Bromoxynil
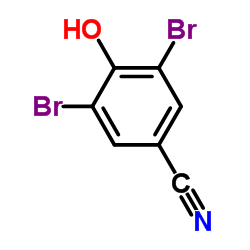
Bromoxynil structure
|
Common Name | Bromoxynil | ||
|---|---|---|---|---|
| CAS Number | 1689-84-5 | Molecular Weight | 276.913 | |
| Density | 2.2±0.1 g/cm3 | Boiling Point | 265.6±40.0 °C at 760 mmHg | |
| Molecular Formula | C7H3Br2NO | Melting Point | 189-191 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 114.4±27.3 °C | |
| Symbol |



GHS06, GHS08, GHS09 |
Signal Word | Danger | |
|
Halogenated pesticide transformation by a laccase-mediator system.
Chemosphere 77(5) , 687-92, (2009) The transformation of organic halogenated pesticides by laccase-mediator system has been investigated. Twelve pesticides were assayed in the presence of nine different mediators. Acetosyringone and syringaldehyde showed to be the best mediators. The halogenat... |
|
|
Microbial degradation of the benzonitrile herbicides dichlobenil, bromoxynil and ioxynil in soil and subsurface environments--insights into degradation pathways, persistent metabolites and involved degrader organisms.
Environ. Pollut. 154(2) , 155-68, (2008) The benzonitriles dichlobenil, bromoxynil and ioxynil are important broad-spectrum or selective herbicides used in agriculture, orchards and public areas worldwide. The dichlobenil metabolite 2,6-dichlorobenzamide is the most frequently encountered groundwate... |
|
|
Analysis of trace levels of pesticides in rainwater by SPME and GC-tandem mass spectrometry after derivatisation with PFBBr.
Anal. Bioanal. Chem 387(1) , 359-68, (2007) Solid-phase microextraction (SPME) was used for the analysis of some pesticides (bromoxynil, chlorotoluron, diuron, isoproturon, 2,4-MCPA, MCPP and 2,4-D) in rainwater after derivatisation with PFBBr and gas chromatography-ion trap mass spectrometry. The deri... |
|
|
Unique cellular effect of the herbicide bromoxynil revealed by electrophysiological studies using characean cells.
J. Plant Res. 123(5) , 715-22, (2010) In a previous paper, we proposed that the primary action of the herbicide bromoxynil (BX; 3,5-dibromo-4-hydroxybenzonitrile) is cytosol acidification, based on the fact that bromoxynil induced the inhibition of cytoplasmic streaming and cell death of Chara co... |
|
|
Demonstrating formation of potentially persistent transformation products from the herbicides bromoxynil and ioxynil using liquid chromatography-tandem mass spectrometry (LC-MS/MS).
Pest Manag. Sci. 63(2) , 141-9, (2007) It is shown that potentially persistent transformation products can be formed from the herbicides bromoxynil (3,5-dibromo-4-hydroxybenzonitrile) and ioxynil (3,5-diiodo-4-hydroxybenzonitrile), and possible leaching to groundwater is discussed. A similar proce... |
|
|
Dissipation of six acid herbicides in water and sediment of two Canadian prairie wetlands.
Environ. Toxicol. Chem. 30(9) , 1982-9, (2011) In the present study, an ephemeral (E) and a semipermanent (SP) wetland were divided into halves using a polyvinyl curtain and one-half of each wetland was treated with dicamba (3,6-dichloro-o-anisic acid), bromoxynil (3,5-dibromo-4-hydroxy-benzonitrile), MCP... |
|
|
Further electrophysiological studies on cellular effect of herbicide, bromoxynil, using characean cells.
J. Plant Res. 125(6) , 749-54, (2012) In the previous paper, I reported that 3,5-dibromo-4-hydroxybenzonitrile (bromoxynil) depolarizes the plasma membrane by inhibiting the electrogenic proton pump and discussed that the inhibition is caused by cytosol acidification due to influx of protonated b... |
|
|
Photosensitized degradation in water of the phenolic pesticides bromoxynil and dichlorophen in the presence of riboflavin, as a model of their natural photodecomposition in the environment.
J. Hazard. Mater. 186(1) , 466-72, (2011) Within the context of environmentally friendly methods for the elimination of surface-water pollutants, the photodegradation of the phenolic pesticides bromoxynil (BXN) and dichlorophen (DCP) under simulated natural conditions has been studied. The work was d... |
|
|
Degradation of bromoxynil and trifluralin in natural water by direct photolysis and UV plus H(2)O(2) advanced oxidation process.
Water Res. 44(7) , 2221-8, (2010) The degradation of two pesticides, bromoxynil and trifluralin, was investigated in ultrapure and natural water solutions under ultraviolet (UV) light and a combination of UV and hydrogen peroxide (H(2)O(2)). The effect of pH on the photooxidation of the pesti... |
|
|
Oxidation kinetics of two pesticides in natural waters by ozonation and ozone combined with hydrogen peroxide.
Water Res. 45(8) , 2517-26, (2011) The oxidation of bromoxynil and trifluralin was investigated using ozone (O(3)) and O(3) combined with hydrogen peroxide (H(2)O(2)) in natural waters using batch reactors. The results indicated that these pesticides could not be completely degraded during ozo... |