Rhodanine
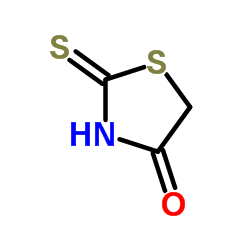
Rhodanine structure
|
Common Name | Rhodanine | ||
|---|---|---|---|---|
| CAS Number | 141-84-4 | Molecular Weight | 133.192 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 218.2ºC at 760mmHg | |
| Molecular Formula | C3H3NOS2 | Melting Point | 165-169 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 85.7ºC | |
| Symbol |


GHS05, GHS07 |
Signal Word | Danger | |
|
Biochemical and histopathological evidence on the beneficial effects of Tragopogon graminifolius in TNBS-induced colitis.
Pharm. Biol. 53(3) , 429-36, (2015) Tragopogon graminifolius DC. (Compositae) (TG) has been proposed as an efficacious remedy for gastrointestinal ulcers in Iranian traditional medicine.The present study evaluates the efficacy of TG on experimental colitis and the responsible mechanisms.After i... |
|
|
Acidified bile acids increase hTERT expression via c-myc activation in human gastric cancer cells.
Oncol. Rep. 33 , 3038-44, (2015) Human telomerase reverse transcriptase (hTERT) is upregulated in most cancer cell types as well in immortalized cells. The underlying mechanism for such upregulation, however, remains largely unknown. We report here that bile acids under acidified media incre... |
|
|
Disruption of copper-dependent signaling pathway in the nitrofen-induced congenital diaphragmatic hernia.
Pediatr. Surg. Int. 31(1) , 31-5, (2015) Normal development of the fetal diaphragm requires muscularization of the diaphragm as well as the structural integrity of its underlying connective tissue components. Developmental mutations that inhibit the formation of extracellular matrix (ECM) have been ... |
|
|
Inhibitor selectivity between aldo–keto reductase superfamily members AKR1B10 and AKR1B1: Role of Trp112 (Trp111)
FEBS Lett. 587(22) , 3681-6, (2013) • Crystal structures of AKR1B10 holoenzyme. • Trp112 side-chain flipping in AKR1B10 explains non-selectivity of AKR1B1 inhibitors. • Native Trp112 side-chain orientation is critical for selective AKR1B10 inhibitors. |
|
|
Development of an ESI-LC-MS-based assay for kinetic evaluation of Mycobacterium tuberculosis shikimate kinase activity and inhibition.
Anal. Chem. 87(4) , 2129-36, (2015) A simple and reliable liquid chromatography-mass spectrometry (LC-MS) assay has been developed and validated for the kinetic characterization and evaluation of inhibitors of shikimate kinase from Mycobacterium tuberculosis (MtSK), a potential target for the d... |
|
|
Versatile routes to marine sponge metabolites through benzylidene rhodanines.
Org. Lett. 14(24) , 6310-3, (2012) The first total synthesis of the marine natural products Psammaplin C and Tokaradine A is described. Benzylidene rhodanines were utilized as versatile intermediates toward the synthesis of seven brominated marine sponge metabolites through the optimization of... |
|
|
Structural determinants of Tau aggregation inhibitor potency.
J. Biol. Chem. 288(45) , 32599-611, (2013) Small-molecule Tau aggregation inhibitors are under investigation as potential therapeutic agents against Alzheimer disease. Many such inhibitors have been identified in vitro, but their potency-driving features, and their molecular targets in the Tau aggrega... |
|
|
Undecaprenyl diphosphate synthase inhibitors: antibacterial drug leads.
J. Med. Chem. 57(13) , 5693-701, (2014) There is a significant need for new antibiotics due to the rise in drug resistance. Drugs such as methicillin and vancomycin target bacterial cell wall biosynthesis, but methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococci (... |
|
|
SAR and 3D-QSAR studies on thiadiazolidinone derivatives: exploration of structural requirements for glycogen synthase kinase 3 inhibitors.
J. Med. Chem. 48 , 7103-12, (2005) The 2,4-disubstituted thiadiazolidinones (TDZD) are described as the first ATP-noncompetitive GSK-3 inhibitors. Following an SAR study about TDZD, different structural modifications in the heterocyclic ring aimed to test the influence of each heteroatom on th... |
|
|
Privileged scaffolds or promiscuous binders: a comparative study on rhodanines and related heterocycles in medicinal chemistry.
J. Med. Chem. 55 , 743-53, (2012) Rhodanines and related five-membered heterocycles with multiple heteroatoms have recently gained a reputation of being unselective compounds that appear as "frequent hitters" in screening campaigns and therefore have little value in drug discovery. However, t... |