3-formyl-6-methylchromone
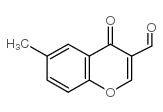
3-formyl-6-methylchromone structure
|
Common Name | 3-formyl-6-methylchromone | ||
|---|---|---|---|---|
| CAS Number | 42059-81-4 | Molecular Weight | 188.17900 | |
| Density | 1.364g/cm3 | Boiling Point | 335.1ºC at 760 mmHg | |
| Molecular Formula | C11H8O3 | Melting Point | 172-173 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 150.3ºC | |
|
Multidrug resistance reversal by 3-formylchromones in human colon cancer and human mdr1 gene-transfected mouse lymphoma cells.
In Vivo 20(5) , 645-9, (2006) Several new 3-formylchromone derivatives proved to be modifiers of multidrug resistance in mouse lymphoma cells and in human Colo320 colon cancer cells. There is apparently a structure-activity relationship between the antiproliferative multidrug resistance-r... |
|
|
Carbonic anhydrase inhibitors. Inhibition of cytosolic/tumor-associated carbonic anhydrase isozymes I, II, IX, and XII with Schiff's bases incorporating chromone and aromatic sulfonamide moieties, and their zinc complexes.
Bioorg. Med. Chem. Lett. 15(12) , 3096-101, (2005) A series of Schiff's bases was prepared by reaction of 3-formyl-chromone or 6-methyl-3-formyl-chromone with aromatic sulfonamides, such as sulfanilamide, homosulfanilamide, 4-aminoethyl-benzenesulfonamide, a pyrimidinyl-substituted sulfanilamide derivative, s... |
|
|
Enthalpies of combustion and formation of 3-formylchromones. Flores H, et al.
Thermochim. Acta 450(1) , 35-37, (2006)
|
|
|
Synthetic approach for novel bis (a-aminophosphonic acid) derivatives of chromone containing 1, 2, 4, 3-triazaphosphole moieties Ali, TE and Halacheva, SS.
Heteroatom Chem. 20(3) , 117-22, (2009)
|
|
|
Synthesis, characterization, crystal structure and antimicrobial activity of copper (II) complexes with a thiosemicarbazone derived from 3-formyl-6-methylchromone. Ilies DC, et al.
Polyhedron 81 , 123-131, (2014)
|