2-Methyl-2-propanyl (2-oxoethyl)carbamate
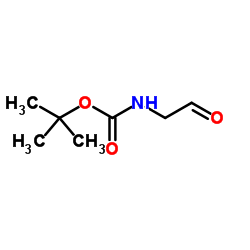
2-Methyl-2-propanyl (2-oxoethyl)carbamate structure
|
Common Name | 2-Methyl-2-propanyl (2-oxoethyl)carbamate | ||
|---|---|---|---|---|
| CAS Number | 89711-08-0 | Molecular Weight | 159.183 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 237.2±23.0 °C at 760 mmHg | |
| Molecular Formula | C7H13NO3 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 97.3±22.6 °C | |
|
Synthesis of fused heteroarylprolines and pyrrolopyrroles.
J. Org. Chem. 69 , 4656-4662, (2004) Fused heteroarylprolines were prepared starting from 4-oxo-N-(PhF)proline benzyl ester (6, PhF = 9-(9-phenylfluorenyl)) following two approaches. First, allylation of oxoproline 6 followed by Wacker oxidation gave 1,4-dione 8 that was selectively converted to... |
|
|
Tumor-targeting of EGFR inhibitors by hypoxia-mediated activation.
Angew. Chem. Int. Ed. Engl. 53(47) , 12930-5, (2014) The development of receptor tyrosine-kinase inhibitors (TKIs) was a major step forward in cancer treatment. However, the therapy with TKIs is limited by strong side effects and drug resistance. The aim of this study was the design of novel epidermal growth fa... |
|
|
Single cell imaging of Bruton's tyrosine kinase using an irreversible inhibitor.
Sci. Rep. 4 , 4782, (2014) A number of Bruton's tyrosine kinase (BTK) inhibitors are currently in development, yet it has been difficult to visualize BTK expression and pharmacological inhibition in vivo in real time. We synthesized a fluorescent, irreversible BTK binder based on the d... |
|
|
Efficient total synthesis of (+)-negamycin, a potential chemotherapeutic agent for genetic diseases.
Chem. Commun. (Camb.) (20) , 2379-81, (2008) Herein, we describe an efficient strategy for the total synthesis of (+)-negamycin using commercially available achiral N-Boc-2-aminoacetaldehyde as starting material with 42% overall yield for a limited number of steps. |
|
|
Mild organocatalytic alpha-methylenation of aldehydes.
J. Org. Chem. 71 , 2538, (2006) A rapid and extremely convenient method for alpha-methylenation of aldehydes with aqueous formaldehyde is described. Two optimal catalytic systems are presented that allow short reaction times and afford the functionalized products in good to excellent yields... |
|
|
J. Org. Chem. 70 , 10869, (2005)
|
|
|
Diethyl [3-Cyano-2-Oxo-3-(Triphenylphosphoranylidene) propyl] phosphonate: A Useful Horner-Wadsworth-Emmons Reagent for alpha-Keto (Cyanomethylene)-triphenylphosphoranes from Carbonyl Compounds. Lee K.
Bull. Korean Chem. Soc. 28(10) , 1641, (2007)
|