Nitromide
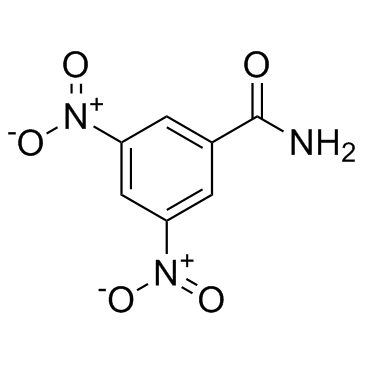
Nitromide structure
|
Common Name | Nitromide | ||
|---|---|---|---|---|
| CAS Number | 121-81-3 | Molecular Weight | 211.13200 | |
| Density | 1.601g/cm3 | Boiling Point | 314.5ºC at 760mmHg | |
| Molecular Formula | C7H5N3O5 | Melting Point | 183-185 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 144ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Highly sensitive voltammetric sensor based on catechol-derivative-multiwall carbon nanotubes for the catalytic determination of captopril in patient human urine samples.
Colloids Surf. B Biointerfaces 87(2) , 480-8, (2011) A new catechol-derivative compound, N-(3,4-dihydroxyphenethyl)-3,5-dinitrobenzamide, was synthesized and used to construct a modified-carbon nanotubes paste electrode. The electro-oxidation of captopril at the surface of the modified electrode was studied usi... |
|
|
Preparative enantioseparation of (+/-)-N-(3,4-cis-3-decyl-1,2,3,4-tetrahydrophenanthren-4-yl)-3,5-dinitrobenzamide by centrifugal partition chromatography.
J. Chromatogr. A. 1217(8) , 1183-90, (2010) The racemic compound (+/-)-N-(3,4-cis-3-decyl-1,2,3,4-tetrahydrophenanthren-4-yl)-3,5-dinitrobenzamide ((+/-)-1), an analogue of increased lipophilicity of the chiral selector (CS) contained in the Whelk-O HPLC chiral stationary phase (CSP) has been resolved ... |
|
|
Metabolism of nitromide in the rat. II. Sites of nitro-reduction.
Xenobiotica 10(4) , 299-305, (1980) 1. Nitromide (3,5-dinitrobenzamide) is reduced to monoamino- and diamino-metabolites in vitro on anaerobic incubation with rat intestinal microflora. This conversion is suppressed by the antibiotics neomycin, tetracycline and bacitracin. 2. Nitromide is also ... |
|
|
Identification of the major urinary and fecal metabolites of 3,5-dinitrobenzamide in chickens and rats.
Chemosphere 38(8) , 1757-62, (1999) Colostomized chickens given oral doses of 3,5-dinitrobenzamide (nitromide) cleared nitromide predominantly through the urine (58% of dose) and feces (21% of dose). Rats cleared 52% of nitromide via urinary excretion and 44% via feces. Major urinary metabolite... |
|
|
The metabolic fate of nitromide in the rat. I. Metabolism and excretion.
Xenobiotica 10(4) , 289-97, (1980) 1. Following oral administration of nitromide (3,5-dinitrobenzamide) to rats, 67.9% of the dose was excreted in urine and 32.6% in the faeces in 96 h. Significant biliary excretion of nitromide metabolites also occurred, although no evidence of enterohepatic ... |