1,2-Diphenylethanamine
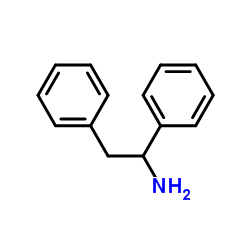
1,2-Diphenylethanamine structure
|
Common Name | 1,2-Diphenylethanamine | ||
|---|---|---|---|---|
| CAS Number | 25611-78-3 | Molecular Weight | 197.276 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 311.1±0.0 °C at 760 mmHg | |
| Molecular Formula | C14H15N | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 136.8±10.9 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Lefetamine, a controlled drug and pharmaceutical lead of new designer drugs: synthesis, metabolism, and detectability in urine and human liver preparations using GC-MS, LC-MS(n), and LC-high resolution-MS/MS.
Anal. Bioanal. Chem 407(6) , 1545-57, (2015) Lefetamine (N,N-dimethyl-1,2-diphenylethylamine, L-SPA) was marketed as an opioid analgesic in Japan and Italy. After being widely abused, it became a controlled substance. It seems to be a pharmaceutical lead for designer drugs because N-ethyl-1,2-diphenylet... |
|
|
Superficially porous particles vs. fully porous particles for bonded high performance liquid chromatographic chiral stationary phases: isopropyl cyclofructan 6.
J. Chromatogr. A. 1363 , 89-95, (2014) This work reports a comparison of HPLC separations of enantiomers with chiral stationary phases (CSPs) prepared by chemically bonding cyclofructan-6, functionalized with isopropyl carbamate groups on fully and superficially porous particles (SPPs). The chroma... |
|
|
NMDA receptor affinities of 1,2-diphenylethylamine and 1-(1,2-diphenylethyl)piperidine enantiomers and of related compounds.
Bioorg. Med. Chem. 17 , 3456-62, (2009) We resolved 1,2-diphenylethylamine (DPEA) into its (S)- and (R)-enantiomer and used them as precursors for synthesis of (S)- and (R)-1-(1,2-diphenylethyl)piperidine, flexible homeomorphs of the NMDA channel blocker MK-801. We also describe the synthesis of th... |