phenyl-(3-phenyloxiran-2-yl)methanone
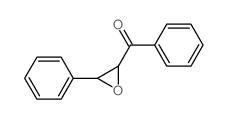
phenyl-(3-phenyloxiran-2-yl)methanone structure
|
Common Name | phenyl-(3-phenyloxiran-2-yl)methanone | ||
|---|---|---|---|---|
| CAS Number | 7570-86-7 | Molecular Weight | 224.25500 | |
| Density | 1.209g/cm3 | Boiling Point | 374.1ºC at 760 mmHg | |
| Molecular Formula | C15H12O2 | Melting Point | 86-89ºC(lit.) | |
| MSDS | USA | Flash Point | 174ºC | |
|
Highly enantioselective epoxidation of 2,4-diarylenones by using dimeric cinchona phase-transfer catalysts: enhancement of enantioselectivity by surfactants.
Angew. Chem. Int. Ed. Engl. 44(9) , 1383-5, (2005)
|
|
|
Anti-inflammatory effects of trans-1,3-diphenyl-2,3-epoxypropane-1-one mediated by suppression of inflammatory mediators in LPS-stimulated RAW 264.7 macrophages.
Food Chem. Toxicol. 53 , 371-5, (2013) To assess the potential therapeutic properties of trans-1,3-diphenyl-2,3-epoxypropane-1-one (DPEP), its anti-inflammatory effects were investigated in lipopolysaccharide (LPS)-stimulated mouse macrophage (RAW 264.7) cells. DPEP induced dose-dependent reductio... |
|
|
Structure-mutagenicity relationships of chalcones and their oxides in the Salmonella assay.
Mutat. Res. 169(3) , 71-9, (1986) 31 p-monosubstituted chalcones (E-1, 3-diphenylpropene-1-one) and the corresponding oxides (E-1-benzoyl-2-phenyloxirane) were tested for mutagenic activity on two strains of Salmonella typhimurium (TA98 and TA100) with and without rat liver microsomal and cyt... |
|
|
C(alpha)-tetrasubstituted amino acid based peptides in asymmetric catalysis.
Biopolymers 84(1) , 97-104, (2006) C(alpha)-tetrasubstituted alpha-amino acids constitute a powerful tool for controlling the conformation of short peptide sequences. Chiral peptides may be used in stereoselective reactions both for asymmetric induction and in kinetic resolution. By reviewing ... |
|
|
Cell cycle arrest and apoptosis induction by an anticancer chalcone epoxide.
Arch. Pharm. (Weinheim) 343(8) , 429-39, (2010) Safe and effective chemotherapeutic agents for the treatment of pancreatic cancer remain elusive. We found that chalcone epoxides (1,3-diaryl-2,3-epoxypropanones) inhibited growth in two pancreatic cancer cell lines, BxPC-3 and MIA PaCa-2. Three compounds wer... |
|
|
Chalcone epoxide intermediates in the syntheses of lignin-related phenylcoumarans.
Acta Crystallogr. C 62(Pt 10) , o625-7, (2006) Compounds (2R*,3S*)-1-(3,4-dimethoxyphenyl)-3-{3-methoxy-2-[(2R*)-tetrahydropyran-2-yloxy]phenyl}-2,3-epoxy-1-propanone, C23H26O7, (I), and trans-1-(3,4-dimethoxyphenyl)-3-[3-methoxy-2-(methoxymethoxy)phenyl]-2,3-epoxy-1-propanone, C20H22O7, (II), were obtain... |
|
|
The atmospheric pressure Meerwein reaction.
J. Mass Spectrom. 41(4) , 470-6, (2006) We have already shown that the in-vacuum gas-phase Meerwein reaction of (thio)acylium ions is general in nature and useful for class-selective screening of cyclic (thio)epoxides. Herein we report that this gas-phase reaction can also be performed efficiently ... |
|
|
The effects of metyrapone, chalcone epoxide, benzil, clotrimazole and related compounds on the activity of microsomal epoxide hydrolase in situ, in purified form and in reconstituted systems towards different substrates.
Eur. J. Biochem. 159(2) , 415-23, (1986) The influence of metyrapone, chalcone epoxide, benzil and clotrimazole on the activity of microsomal epoxide hydrolase towards styrene oxide, benzo[a]pyrene 4,5-oxide, estroxide and androstene oxide was investigated. The studies were performed using liver mic... |
|
|
Benzil, a potent activator of microsomal epoxide hydrolase in vitro.
Eur. J. Biochem. 112(3) , 643-8, (1980) Benzil was found to be a very potent activator of microsomal epoxide hydrolase activity (measured with styrene oxide as substrate) in vitro. The activating effect was uncompetitive and benzil causes approximately ninefold increases in both the apparent V and ... |
|
|
Inhibition of cytosolic epoxide hydrolase by trans-3-phenylglycidols.
Biochem. Pharmacol. 42(6) , 1163-75, (1991) The inhibition of murine cytosolic epoxide hydrolase has been studied with both racemic and enantiomerically pure trans-3-phenylglycidols. These compounds are the first enantioselective, slow binding inhibitors of cytosolic epoxide hydrolase. The (2S,3S)-3-ph... |