C(alpha)-tetrasubstituted amino acid based peptides in asymmetric catalysis.
Giulia Licini, Marcella Bonchio, Quirinus B Broxterman, Bernard Kaptein, Alessandro Moretto, Claudio Toniolo, Paolo Scrimin
Index: Biopolymers 84(1) , 97-104, (2006)
Full Text: HTML
Abstract
C(alpha)-tetrasubstituted alpha-amino acids constitute a powerful tool for controlling the conformation of short peptide sequences. Chiral peptides may be used in stereoselective reactions both for asymmetric induction and in kinetic resolution. By reviewing recent data from our own laboratories and by presenting new results on the stereoselective oxidation of chalcone to the corresponding oxide, this contribution shows that the control of peptide conformation is a critical issue in order to achieve successful stereoselective catalysis.Copyright 2005 Wiley Periodicals, Inc.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
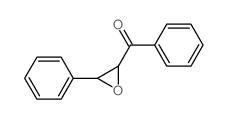 |
phenyl-(3-phenyloxiran-2-yl)methanone
CAS:7570-86-7 |
C15H12O2 |
|
Highly enantioselective epoxidation of 2,4-diarylenones by u...
2005-02-18 [Angew. Chem. Int. Ed. Engl. 44(9) , 1383-5, (2005)] |
|
Anti-inflammatory effects of trans-1,3-diphenyl-2,3-epoxypro...
2013-03-01 [Food Chem. Toxicol. 53 , 371-5, (2013)] |
|
Structure-mutagenicity relationships of chalcones and their ...
1986-03-01 [Mutat. Res. 169(3) , 71-9, (1986)] |
|
Cell cycle arrest and apoptosis induction by an anticancer c...
2010-08-01 [Arch. Pharm. (Weinheim) 343(8) , 429-39, (2010)] |
|
Chalcone epoxide intermediates in the syntheses of lignin-re...
2006-10-01 [Acta Crystallogr. C 62(Pt 10) , o625-7, (2006)] |