Levonorgestrel
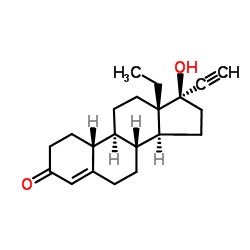
Levonorgestrel structure
|
Common Name | Levonorgestrel | ||
|---|---|---|---|---|
| CAS Number | 797-63-7 | Molecular Weight | 312.446 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 459.1±45.0 °C at 760 mmHg | |
| Molecular Formula | C21H28O2 | Melting Point | 206ºC | |
| MSDS | Chinese USA | Flash Point | 195.4±21.3 °C | |
| Symbol |


GHS07, GHS08 |
Signal Word | Danger | |
|
Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species.
Chem. Res. Toxicol. 23 , 171-83, (2010) Drug-induced liver injury is one of the main causes of drug attrition. The ability to predict the liver effects of drug candidates from their chemical structures is critical to help guide experimental drug discovery projects toward safer medicines. In this st... |
|
|
Translating clinical findings into knowledge in drug safety evaluation--drug induced liver injury prediction system (DILIps).
J. Sci. Ind. Res. 65(10) , 808, (2006) Drug-induced liver injury (DILI) is a significant concern in drug development due to the poor concordance between preclinical and clinical findings of liver toxicity. We hypothesized that the DILI types (hepatotoxic side effects) seen in the clinic can be tra... |
|
|
Developing structure-activity relationships for the prediction of hepatotoxicity.
Chem. Res. Toxicol. 23 , 1215-22, (2010) Drug-induced liver injury is a major issue of concern and has led to the withdrawal of a significant number of marketed drugs. An understanding of structure-activity relationships (SARs) of chemicals can make a significant contribution to the identification o... |
|
|
A predictive ligand-based Bayesian model for human drug-induced liver injury.
Drug Metab. Dispos. 38 , 2302-8, (2010) Drug-induced liver injury (DILI) is one of the most important reasons for drug development failure at both preapproval and postapproval stages. There has been increased interest in developing predictive in vivo, in vitro, and in silico models to identify comp... |
|
|
An updated steroid benchmark set and its application in the discovery of novel nanomolar ligands of sex hormone-binding globulin.
J. Med. Chem. 51 , 2047-56, (2008) A benchmark data set of steroids with known affinity for sex hormone-binding globulin (SHBG) has been widely used to validate popular molecular field-based QSAR techniques. We have expanded the data set by adding a number of nonsteroidal SHBG ligands identifi... |
|
|
Impact of induced fit on ligand binding to the androgen receptor: a multidimensional QSAR study to predict endocrine-disrupting effects of environmental chemicals.
J. Med. Chem. 48 , 5666-74, (2005) We investigated the influence of induced fit of the androgen receptor binding pocket on free energies of ligand binding. On the basis of a novel alignment procedure using flexible docking, molecular dynamics simulations, and linear-interaction energy analysis... |
|
|
Neural computational prediction of oral drug absorption based on CODES 2D descriptors.
Eur. J. Med. Chem. 45 , 930-40, (2010) A neural model based on a numerical molecular representation using CODES program to predict oral absorption of any structure is described. This model predicts both high and low-absorbed compounds with a global accuracy level of 74%. CODES/ANN methodology show... |
|
|
Hologram QSAR model for the prediction of human oral bioavailability.
Bioorg. Med. Chem. 15 , 7738-45, (2007) A drug intended for use in humans should have an ideal balance of pharmacokinetics and safety, as well as potency and selectivity. Unfavorable pharmacokinetics can negatively affect the clinical development of many otherwise promising drug candidates. A varie... |
|
|
Multi-residue analytical methodology-based liquid chromatography-time-of-flight-mass spectrometry for the analysis of pharmaceutical residues in surface water and effluents from sewage treatment plants and hospitals.
J. Chromatogr. A. 1345 , 139-53, (2014) An analytical method that facilitated the analysis of 11 pharmaceuticals residue (caffeine, prazosin, enalapril, carbamazepine, nifedipine, levonorgestrel, simvastatin, hydrochlorothiazide, gliclazide, diclofenac-Na, and mefenamic acid) with a single pre-trea... |
|
|
Responses to various exposure durations of levonorgestrel during early-life stages of fathead minnows (Pimephales promelas).
Aquat. Toxicol. 161 , 33-40, (2015) Pharmaceuticals are routinely detected in the environment; and several of these compounds have been extensively researched due to their potential impacts to the endocrine system of aquatic organisms. The negative reproductive consequences of synthetic progest... |