Hordenine
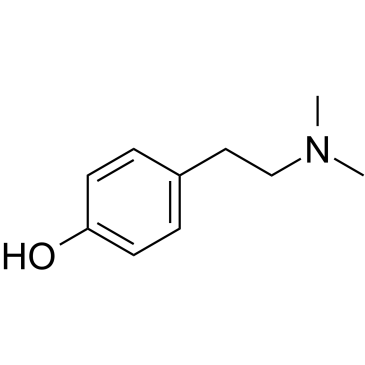
Hordenine structure
|
Common Name | Hordenine | ||
|---|---|---|---|---|
| CAS Number | 539-15-1 | Molecular Weight | 165.232 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 270.2±23.0 °C at 760 mmHg | |
| Molecular Formula | C10H15NO | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 123.5±21.3 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Development of immunoassays for tyramine and tryptamine toxins of Phalaris aquatica L.
J. Agric. Food Chem. 48(1) , 27-32, (2000) The leaves of the perennial pasture grass Phalaris aquatica L. (phalaris) contain two groups of known toxins, indole alkaloids, primarily dimethyltryptamines and N-methyltyramines, which cause illnesses in grazing animals, especially sheep. Using amino-reacti... |
|
|
Some hematological and histopathological effects of the alkaloids gramine and hordenine on meadow voles (Microtus pennsylvanicus).
Toxicology 18(2) , 125-31, (1980) Meadow voles (Microtus pennsylvanicus) were used to evaluate the relative toxicity of the alkaloids, gramine and hordenine, which are present in reed canarygrass (Phalaris arundinacea) and to assess their effects on the quality of this grass as a forage. One ... |
|
|
Rapid formation of N-nitrosodimethylamine from gramine, a naturally occurring precursor in barley malt.
IARC Sci. Publ. (57) , 337-46, (1984) The two tertiary amine alkaloids, hordenine and gramine, which are biosynthesized in malt during germination, were subjected to nitrosation under conditions typical for the study of tertiary amine nitrosation. At 65 degrees C in dilute aqueous acid (pH 4.4 or... |
|
|
N-nitrosodimethylamine precursors in malt.
IARC Sci. Publ. (41) , 71-80, (1982) NDMA is formed in malt because NOx reacts with certain amines in germinated barley when it is kilned. Hordenine is the major precursor of NDMA, although gramine and sarcosine can possibly contribute minor amounts. The hordenine is formed in the developing see... |
|
|
Hydroxylating activity of tyrosinase and its dependence on hydrogen peroxide.
Arch. Biochem. Biophys. 373(1) , 255-60, (2000) The aim of this work was to study the hydroxylation of N, N-dimethyltyramine (DMTA) by tyrosinase in the presence of hydrogen peroxide, a reaction that does not take place without the addition of the hydrogen peroxide. Some properties of this hydroxylating ac... |
|
|
Deamination of hordenine by monoamine oxidase and its action on vasa deferentia of the rat.
J. Pharm. Pharmacol. 41(6) , 421-3, (1989) The selectivity of the naturally occurring amine, N,N-dimethyltyramine (hordenine) for monoamine oxidase (MAO) and its action upon isolated vasa deferentia of the rat was investigated. Hordenine was deaminated by rat liver MAO with a Michaelis constant of 479... |
|
|
Phenylalkylamine alkaloids from Stapelia hirsuta L.
Nat. Prod. Res. 20(8) , 710-4, (2006) Four alkaloids of the phenethylamine derivatives have been isolated from the n-butanol fraction of the aerial parts of Stapelia hirsuta L. The structures of the isolated alkaloids were determined as N-acetyl hordenine (a new natural compound), hordenine, cand... |
|
|
[Not Available].
C. R. Seances. Soc. Biol. Fil. 139 , 630, (1945)
|
|
|
[Effect of hypocapnic anoxemia on hordenine induced vasoconstriction].
C. R. Seances. Soc. Biol. Fil. 144(23-24) , 1687-89, (1950)
|
|
|
Separation of two distinct S-adenosylmethionine dependent N-methyltransferases involved in hordenine biosynthesis in Hordeum vulgare.
Plant Cell Rep. 1 , 236-239, (1982) Hordenine is biosynthesized in young roots of barley by subsequent N-methylation of tyramine. It was shown that two distinct enzymes are responsible for these methylation reactions. They differed in their pH-optimum, their stability in dependence of the pH-va... |