quinidine gluconate
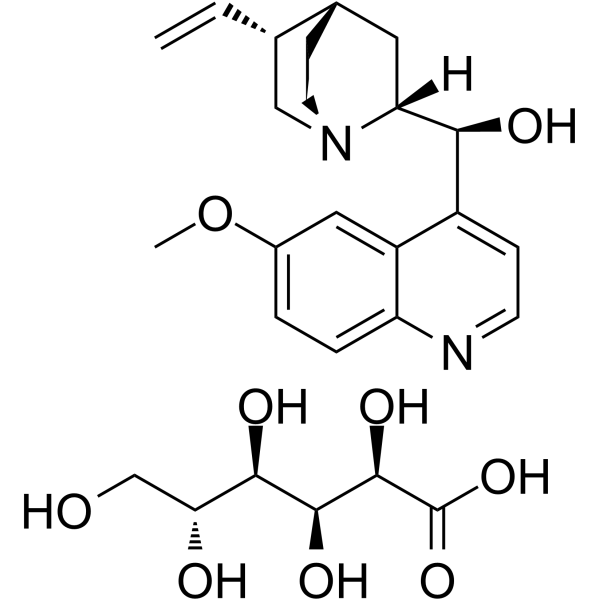
quinidine gluconate structure
|
Common Name | quinidine gluconate | ||
|---|---|---|---|---|
| CAS Number | 7054-25-3 | Molecular Weight | 520.57200 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C26H36N2O9 | Melting Point | 175-176ºC | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Disposition of 3-hydroxyquinidine in patients receiving initial intravenous quinidine gluconate for electrophysiology testing of ventricular tachycardia.
DICP 23(5) , 375-8, (1989) The formation rate constant and elimination rate constant for 3-hydroxyquinidine were determined in eight patients with ventricular tachycardia. These two parameters (mean +/- SD) were found to be 0.784 +/- 0.202 and 0.042 +/- 0.058 h-1, respectively. Coeffic... |
|
|
Quinidine-induced rheumatic syndromes.
Semin. Arthritis Rheum. 24(5) , 315-22, (1995) Quinidine is a commonly used antiarrhythmic agent that is rarely associated with rheumatologic toxicity. However, quinidine-induced lupus, antinuclear antibody negative lupus-like syndrome, polymyalgia rheumatica-like illness, muscle weakness, and isolated cr... |
|
|
Transfusion-transmitted malaria in a kidney transplant recipient. How safe is our blood transfusion?
Saudi Med. J. 29(2) , 293-5, (2008) A 51-year-old male patient with living, unrelated kidney transplantation in Iran in June 2001, developed Plasmodium falciparum P. falciparum infection. He was maintained on cyclosporine A, mycophenolate mofetil, and prednisone. In August 2005, he was admitted... |
|
|
Prospective comparison of intravenous quinidine and intravenous procainamide in patients undergoing electrophysiologic testing.
Am. Heart J. 136(1) , 49-56, (1998) Intravenous procainamide hydrochloride is frequently used in the acute care setting and during electrophysiologic testing, but intravenous quinidine gluconate is rarely used because of concerns about its safety. This study prospectively compares the hemodynam... |
|
|
Effect of dietary fat content on the bioavailability of a sustained release quinidine gluconate tablet.
Biopharm. Drug Dispos. 11(1) , 17-29, (1990) Quinidine gluconate 324 mg sustained release tablets (Quinaglute) was administered as a single dose to 15 healthy male subjects following an overnight fast, immediately following a high fat (HF) breakfast or immediately following a low fat (LF) breakfast. Ser... |
|
|
A 21-year-old woman from Africa with fever, anemia, and jaundice 7 days after arriving in the US.
J. Emerg. Nurs. 32(1) , 51-3, (2006)
|
|
|
Loss of quinidine gluconate injection in a polyvinyl chloride infusion system.
Am. J. Health Syst. Pharm. 53(6) , 655-8, (1996) The effect of a polyvinyl chloride (PVC) i.v. administration system on the availability of quinidine gluconate was studied. Quinidine gluconate diluted in 5% dextrose injection was administered intravenously to five healthy volunteers via conventional PVC inf... |
|
|
The treatment of malaria.
N. Engl. J. Med. 336(10) , 733; author reply 733-4, (1997)
|
|
|
Malaria and hereditary elliptocytosis.
Am. J. Hematol. 83(9) , 753, (2008)
|
|
|
Quinidine-induced gastric ulcer.
Gastrointest. Endosc. 60(3) , 481-3, (2004)
|