Z-D-Phe-OH
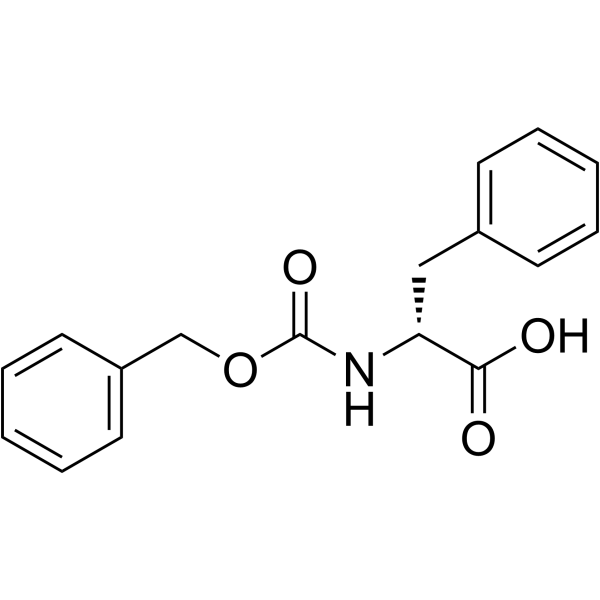
Z-D-Phe-OH structure
|
Common Name | Z-D-Phe-OH | ||
|---|---|---|---|---|
| CAS Number | 2448-45-5 | Molecular Weight | 299.321 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 511.5±50.0 °C at 760 mmHg | |
| Molecular Formula | C17H17NO4 | Melting Point | 80-82ºC | |
| MSDS | Chinese USA | Flash Point | 263.1±30.1 °C | |
|
Effects of metalloendoproteinase inhibitors on secretion and intracellular free calcium in bovine adrenal chromaffin cells.
Biochim. Biophys. Acta 889(1) , 1-5, (1986) The possible role of metalloendoproteinase in stimulus-secretion coupling in adrenal chromaffin cells was examined using the metalloendoproteinase inhibitors 1,10-phenanthroline and carbobenzoxy-Gly-Phe-NH2. Catecholamine release elicited by nicotine or by de... |
|
|
Mechanism of the binding of Z-L-tryptophan and Z-L-phenylalanine to thermolysin and stromelysin-1 in aqueous solutions.
Biochim. Biophys. Acta 1824(2) , 303-10, (2012) The chemical shift of the carboxylate carbon of Z-tryptophan is increased from 179.85 to 182.82 ppm and 182.87 ppm on binding to thermolysin and stromelysin-1 respectively. The chemical shift of Z-phenylalanine is also increased from 179.5 ppm to 182.9 ppm on... |
|
|
Modification of a zinc proteinase from Bacillus mesentericus strain 76 by diethylpyrocarbonate.
Int. J. Pept. Protein Res. 37(4) , 325-30, (1991) Diethylpyrocarbonate (DEPC) inactivated the neutral zinc proteinase from Bacillus mesentericus strain 76/Bacillus subtilis (MCP 76) by ethoxycarbonylation completely. Exposure of the enzyme to DEPC together with the competitive inhibitor Z-L-phenylalanine pre... |
|
|
Use of Z-amino acid-glyceryl esters in protease catalyzed peptide synthesis.
Biomed. Biochim. Acta 50(10-11) , S90-3, (1991) The alpha-glyceryl esters of Z-Gly, Z-Phe and Z-Tyr were synthesized and their use for protease catalyzed peptide synthesis was studied. Three enzymes isolated from crude papain were compared in their catalytic potency. Syntheses with alpha-chymotrypsin were ... |
|
|
Synthesis, characterization and antimicrobial activity of Co(II), Zn(II) and Cd(II) complexes withN-benzyloxycarbonyl-S-phenylalanine
Eur. J. Med. Chem. 44 , 1537-44, (2009) In this paper the first complexes of M 2+ ions (M 2+ = Zn 2+, Cd 2+ and Co 2+) with N-benzyloxycarbonyl- S-phenylalaninato ligand ( 1– 3) are described. The new complexes were characterized by means of elemental analysis, IR and UV–vis spectroscopy, molar con... |
|
|
Solution conformation of the thermolysin inhibitors carbobenzoxy-L-phenylalanine and beta-phenylpropionyl-L-phenylalanine and comparison of the solution conformation to the enzyme-bound conformation.
J. Med. Chem. 25 , 403, (1982) The conformations of enzyme inhibitors in solution and bound to the enzyme thermolysin are investigated as a convenient model for understanding the relationship between the conformation of drugs in solution and at the receptor. The solution conformations of c... |
|
|
Extracellular fibrinogenolytic enzyme of Aspergillus fumigatus: substrate-dependent variations in the proteinase synthesis and characterization of the enzyme.
FEMS Immunol. Med. Microbiol. 7 , 81-91, (1993) To get a better understanding of the role of the previously reported fibrinogenolytic enzyme of Aspergillus fumigatus, we investigated the in vitro conditions of enzyme synthesis and attempted to characterize it. Modification of the nitrogen source did not in... |
|
|
Selective suppression of cathepsin L results from elevations in lysosomal pH and is followed by proteolysis of tau protein.
Neuroreport 9 , 2089-2094, (1998) Incubation of cultured hippocampal slices with chloroquine, a compound that increases the pH of acidic subcellular organelles, for 10 h reduced the activity of cathepsin L by 83 +/- 0.87% (mean +/- s.e.m.) while only marginally suppressing cathepsin B. This e... |