4-(Dimethylamino)phenylboronic acid
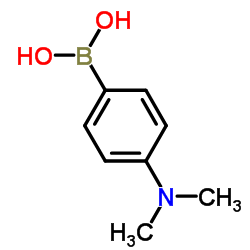
4-(Dimethylamino)phenylboronic acid structure
|
Common Name | 4-(Dimethylamino)phenylboronic acid | ||
|---|---|---|---|---|
| CAS Number | 28611-39-4 | Molecular Weight | 164.997 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 329.9±44.0 °C at 760 mmHg | |
| Molecular Formula | C8H12BNO2 | Melting Point | 227 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 153.3±28.4 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Synthesis and photophysical investigation of a series of push-pull arylvinyldiazine chromophores.
J. Org. Chem. 8th ed., 77 , 4087-4096, (2012) A new series of push-pull arylvinyldiazines has been efficiently prepared by aldol condensation between the appropriate methyldiazine and aromatic aldehyde. The optical absorption and emission properties of these chromophores were studied in different solvent... |
|
|
Nickel-catalyzed Suzuki-Miyaura reaction of aryl fluorides.
J. Am. Chem. Soc. 48th ed., 133 , 19505-19511, (2011) Two protocols for the nickel-catalyzed cross-coupling of aryl fluorides with aryl boronic esters have been developed. The first employs metal fluoride cocatalysts, such as ZrF(4) and TiF(4), which enable Suzuki-Miyaura reactions of aryl fluorides bearing elec... |
|
|
Dramatic Effect of the Gelling Cation on the Catalytic Performances of Alginate-Supported Palladium Nanoparticles for the Suzuki-Miyaura Reaction Chtchigrovsky, M.; et al.
Chem. Mater. 8th ed., 24 , 1505-1510, (2012)
|
|
|
Organic dyes incorporating the cyclopentadithiophene moiety for efficient dye-sensitized solar cells Cheng, X.; et al.
Dyes and Pigments 3rd ed., 92 , 1292-1299, (2012)
|
|
|
Rapid access to 4-substituted-pyrones and 2(5H)-furanones via a palladium-catalyzed C-OH bond activation Hu, Y.; et al.
Tetrahedron 38th ed., 67 , 7258-7262, (2011)
|
|
|
Chiral allene-containing phosphines in asymmetric catalysis.
J. Am. Chem. Soc. 45th ed., 133 , 18066-18069, (2011) We demonstrate that allenes, chiral 1,2-dienes, appended with basic functionality can serve as ligands for transition metals. We describe an allene-containing bisphosphine that, when coordinated to Rh(I), promotes the asymmetric addition of arylboronic acids ... |
|
|
ortho-Phenylene oligomers with terminal push-pull substitution.
Org. Biomol. Chem. 17th ed., 10 , 3398-3405, (2012) ortho-Phenylenes are an emerging class of helical oligomers and polymers. We have synthesized a series of push-pull-substituted o-phenylene oligomers (dimethylamino/nitro) up to the octamer. Conformational analysis of the hexamer using a combination of low-te... |