Furafylline
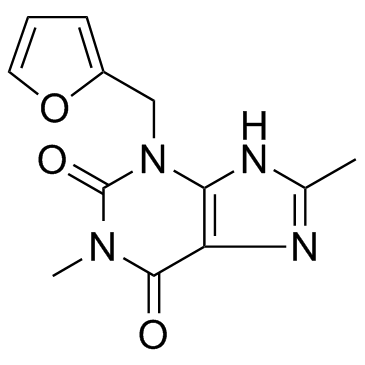
Furafylline structure
|
Common Name | Furafylline | ||
|---|---|---|---|---|
| CAS Number | 80288-49-9 | Molecular Weight | 260.249 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 543.6±60.0 °C at 760 mmHg | |
| Molecular Formula | C12H12N4O3 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 282.6±32.9 °C | |
|
Physicochemical and drug metabolism characterization of a series of 4-aminoquinoline-3-hydroxypyridin-4-one hybrid molecules with antimalarial activity.
Expert Opin. Drug Metab. Toxicol. 10(10) , 1313-24, (2014) Drug resistance by Plasmodium falciparum remains a challenge in malaria chemotherapy. This paper will focus on physicochemical and drug metabolism characterization of a series of 4-aminoquinoline-3-hydroxypyridin-4-one hybrid shown to have antimalarial activi... |
|
|
The cytochrome P450-catalyzed metabolism of levomepromazine: a phenothiazine neuroleptic with a wide spectrum of clinical application.
Biochem. Pharmacol. 90(2) , 188-95, (2014) The aim of the present study was to identify cytochrome P450 isoenzymes (CYPs) involved in the 5-sulfoxidation and N-demethylation of the aliphatic-type phenothiazine neuroleptic levomepromazine in human liver. Experiments were performed in vitro using cDNA-e... |
|
|
Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite.
Drug Metab. Dispos. 38(1) , 92-9, (2010) The aim of the current study is to identify the human cytochrome P450 (P450) isoforms involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. In the in vitro experiments using cDNA-expressed human P4... |
|
|
Chemical inhibitors of CYP450 enzymes in liver microsomes: combining selectivity and unbound fractions to guide selection of appropriate concentration in phenotyping assays.
Xenobiotica 45(2) , 95-106, (2014) 1. Chemical inhibition is the widely used method in reaction phenotyping assays for estimation of specific enzyme contribution to a given metabolic pathway. The results from phenotyping assays depend on the selectivity of chemical inhibitor and the concentrat... |
|
|
Effects of artemisinin antimalarials on Cytochrome P450 enzymes in vitro using recombinant enzymes and human liver microsomes: potential implications for combination therapies.
Xenobiotica 44(7) , 615-26, (2014) 1. Cytochrome P450 enzyme system is the most important contributor to oxidative metabolism of drugs. Modification, and more specifically inhibition, of this system is an important determinant of several drug-drug interactions (DDIs). 2. Effects of the antimal... |
|
|
In vitro metabolism and drug-drug interaction potential of UTL-5g, a novel chemo- and radioprotective agent.
Drug Metab. Dispos. 42(12) , 2058-67, (2014) N-(2,4-dichlorophenyl)-5-methyl-1,2-oxazole-3-carboxamide (UTL-5g), a potential chemo- and radioprotective agent, acts as a prodrug requiring bioactivation to the active metabolite 5-methylisoxazole-3-carboxylic acid (ISOX). UTL-5g hydrolysis to ISOX and 2,4-... |
|
|
Coupling Laser Diode Thermal Desorption with Acoustic Sample Deposition to Improve Throughput of Mass Spectrometry-Based Screening.
J. Biomol. Screen. 21 , 165-75, (2016) The move toward label-free screening in drug discovery has increased the demand for mass spectrometry (MS)-based analysis. Here we investigated the approach of coupling acoustic sample deposition (ASD) with laser diode thermal desorption (LDTD)-tandem mass sp... |
|
|
A comprehensive assay for nine major cytochrome P450 enzymes activities with 16 probe reactions on human liver microsomes by a single LC/MS/MS run to support reliable in vitro inhibitory drug-drug interaction evaluation.
Xenobiotica 45 , 961-77, (2015) 1. A comprehensive method for the simultaneous characterization of xenobiotic compound inhibition of nine major CYP enzymes in human liver microsomes was established by using 16 CYP-catalyzed reactions of 14 probe substrates with three cocktail incubation set... |
|
|
Identification of human cytochrome P450 isozymes involved in the metabolism of naftopidil enantiomers in vitro.
J. Pharm. Pharmacol. 66(11) , 1534-51, (2014) Naftopidil (NAF) is a chiral compound with two enantiomers (R(+)-NAF and S(-)-NAF) and is used as a racemic mixture in clinical practice. This study aims to investigate the metabolism of NAF enantiomers in pooled human liver microsomes (HLMs) and cytochrome P... |
|
|
Preclinical Pharmacokinetics and In Vitro Metabolism of Asunaprevir (BMS-650032), a Potent Hepatitis C Virus NS3 Protease Inhibitor.
J. Pharm. Sci. 104 , 2813-23, (2015) Asunaprevir (ASV; BMS-650032), a low nanomolar inhibitor of the hepatitis C virus (HCV) NS3 protease, is currently under development, in combination with other direct-acting antiviral (DAA) agents for the treatment of chronic HCV infection. Extensive nonclini... |