9-Fluorene acetic acid
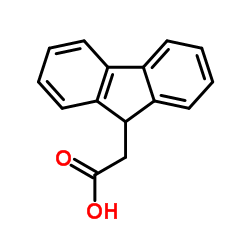
9-Fluorene acetic acid structure
|
Common Name | 9-Fluorene acetic acid | ||
|---|---|---|---|---|
| CAS Number | 6284-80-6 | Molecular Weight | 224.255 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 436.9±14.0 °C at 760 mmHg | |
| Molecular Formula | C15H12O2 | Melting Point | 133-135 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 333.6±15.2 °C | |
|
Structural basis for the potent calpain inhibitory activity of peptidyl alpha-ketoacids.
J. Med. Chem. 51(14) , 4346-50, (2008) A series of peptidyl alpha-ketoacids and alpha-ketoesters was synthesized and studied as mu-calpain inhibitors. Docking studies revealed that the mu-calpain inhibitory activity of the compounds is influenced by hydrogen bonding interactions and the potential ... |
|
|
Prolonged pheromonotropic activity of pseudopeptide mimics of insect pyrokinin neuropeptides after topical application or injection into a moth.
Regul. Pept. 72(2-3) , 161-7, (1997) Amphiphilic pseudopeptide analogs of Phe-Thr-Pro-Arg-Leu-NH2, representing the active C-terminal core pentapeptide of the pyrokinin class of insect neuropeptides, were synthesized by replacement of phenylalanine with hydrocinnamic acid (Hca-Thr-Pro-Arg-Leu-NH... |
|
|
Direct determination of adamantanamine in plasma and urine with automated solid phase derivatization.
J. Chromatogr. A. 619(1) , 93-101, (1993) A simple, highly sensitive and selective method is described for adamantanamine determination in plasma and urine by high-performance liquid chromatography with fluorescence detection. The method involved a simultaneous extraction and derivatization of biolog... |
|
|
Molecular modeling studies of the binding modes of aldose reductase inhibitors at the active site of human aldose reductase.
Bioorg. Med. Chem. 6(10) , 1811-9, (1998) Molecular modeling studies using the CHARMM method have been conducted to study the binding modes of aldose reductase inhibitors at the active site of aldose reductase. The energy minimized structures of aldose reductase with six structurally diverse inhibito... |
|
|
Comparison of 9-fluorenylmethoxycarbonyl and 9-fluoreneacetyl-tagged silica-based derivatization reagents in high-performance liquid chromatography.
J. Pharm. Biomed. Anal. 10(8) , 577-86, (1992) Two silica reagents based on a 4-hydroxy-3-nitrobenzoyl backbone were synthesized and characterized with 9-fluorenylmethoxycarbonyl (FMOC) and 9-fluoreneacetyl (FA) tags. These reagents were tested by derivatization of primary and secondary amines. Derivatiza... |
|
|
Automated HPLC analyses of drugs of abuse via direct injection of biological fluids followed by simultaneous solid-phase extraction and derivatization with fluorescence detection.
Biomed. Chromatogr. 8(2) , 53-62, (1994) An automated system is described for the simultaneous extraction and derivatization of nucleophilic compounds from various biological media. The method includes the use of a solid-phase reagent containing a 9-fluorenylacetate activated ester. The reagent is b... |