| Structure | Name/CAS No. | Articles |
|---|---|---|
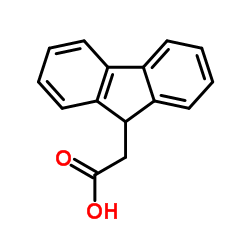 |
9-Fluorene acetic acid
CAS:6284-80-6 |
Isaac O Donkor, Haregewein Assefa, Jiuyu Liu
Index: J. Med. Chem. 51(14) , 4346-50, (2008)
Full Text: HTML
A series of peptidyl alpha-ketoacids and alpha-ketoesters was synthesized and studied as mu-calpain inhibitors. Docking studies revealed that the mu-calpain inhibitory activity of the compounds is influenced by hydrogen bonding interactions and the potential for ionic interaction with active site residues as well as placement of a planar N-terminal capping group into the S 3 pocket of the enzyme.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
9-Fluorene acetic acid
CAS:6284-80-6 |
C15H12O2 |
|
Prolonged pheromonotropic activity of pseudopeptide mimics o...
1997-10-31 [Regul. Pept. 72(2-3) , 161-7, (1997)] |
|
Direct determination of adamantanamine in plasma and urine w...
[J. Chromatogr. A. 619(1) , 93-101, (1993)] |
|
Molecular modeling studies of the binding modes of aldose re...
1998-10-01 [Bioorg. Med. Chem. 6(10) , 1811-9, (1998)] |
|
Comparison of 9-fluorenylmethoxycarbonyl and 9-fluoreneacety...
1992-08-01 [J. Pharm. Biomed. Anal. 10(8) , 577-86, (1992)] |
|
Automated HPLC analyses of drugs of abuse via direct injecti...
1994-01-01 [Biomed. Chromatogr. 8(2) , 53-62, (1994)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved