3,5-Bis(trifluoromethyl)-Phenyl isocyanate
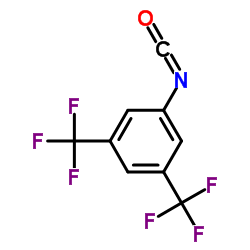
3,5-Bis(trifluoromethyl)-Phenyl isocyanate structure
|
Common Name | 3,5-Bis(trifluoromethyl)-Phenyl isocyanate | ||
|---|---|---|---|---|
| CAS Number | 16588-74-2 | Molecular Weight | 255.117 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 155.4±35.0 °C at 760 mmHg | |
| Molecular Formula | C9H3F6NO | Melting Point | N/A | |
| MSDS | USA | Flash Point | 67.8±0.0 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Determination of accessible amino groups on surfaces by chemical derivatization with 3,5-bis(trifluoromethyl)phenyl isothiocyanate and XPS/NEXAFS analysis.
Anal. Bioanal. Chem 396(2) , 725-38, (2010) The determination of amino groups on surfaces capable of binding biomolecules is important for the understanding and optimization of technologically relevant coupling processes. In this study, three different types of amino-functionalized model surfaces, amin... |
|
|
Zwitterionic salts as mild organocatalysts for transesterification.
Org. Lett. 10(11) , 2187-90, (2008) The exothermic reaction of 3,5-bis(trifluoromethyl)phenyl or 4-nitrophenyl isothiocyanate with 4-pyrrolidinopyridine (PPY) gave the corresponding arylaminothiocarbonylpyridinium salts in quantitative yields. These novel zwitterionic salts were effective as or... |
|
|
1-[3,5-Bis(trifluoro-meth-yl)phen-yl]-3-(2-pyrid-yl)thio-urea.
Acta Crystallogr. Sect. E Struct. Rep. Online 64(5) , o858-o858, (2008) The title compound, C(14)H(9)F(6)N(3)S, exhibits a nearly planar conformation in the solid state, with a dihedral angle between the planes of the benzene and pyridine rings of 14.86 (3)°. The pyridine N atom allows for the formation of a six-membered N-H⋯N(py... |
|
|
Effects of ring substituents on enantioselective recognition of amino alcohols and acids in uryl-based binol receptors. Nandhakumar R, et al.
Tetrahedron 64(33) , 7704-8, (2008)
|