Tafenoquine (Succinate)
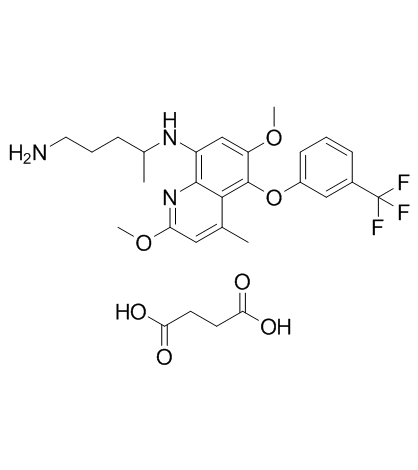
Tafenoquine (Succinate) structure
|
Common Name | Tafenoquine (Succinate) | ||
|---|---|---|---|---|
| CAS Number | 106635-81-8 | Molecular Weight | 581.58100 | |
| Density | 1.237g/cm3 | Boiling Point | 565.6ºC at 760mmHg | |
| Molecular Formula | C28H34F3N3O7 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 295.9ºC | |
|
Uptake of the antileishmania drug tafenoquine follows a sterol-dependent diffusion process in Leishmania.
J. Antimicrob. Chemother. 66(11) , 2562-5, (2011) The present study was designed to elucidate the mechanism of tafenoquine uptake in Leishmania and its sterol dependence.Because tafenoquine is a fluorescent compound, spectrofluorimetric analysis allowed us to monitor its uptake by Leishmania promastigotes an... |
|
|
Transmission-blocking activity of tafenoquine (WR-238605) and artelinic acid against naturally circulating strains of Plasmodium vivax in Thailand.
Am. J. Trop. Med. Hyg. 69(5) , 542-7, (2003) The sporontocidal activity of tafenoquine (WR-238605) and artelinic acid was determined against naturally circulating isolates of Plasmodium vivax in western Thailand. Primaquine was used as a negative control and a dihydroacridine-dione (WR-250547) was used ... |
|
|
Military aviators, special operations forces, and causal malaria prophylaxis.
Mil. Med. 168(12) , 1001-6, (2003) U.S. military aviators are currently restricted to the use of chloroquine or doxycycline for malaria prophylaxis. Ground forces are allowed the additional option of taking mefloquine. These medications are begun before deployment, must be taken for 4 weeks af... |
|
|
A randomized, double-blind, safety and tolerability study to assess the ophthalmic and renal effects of tafenoquine 200 mg weekly versus placebo for 6 months in healthy volunteers.
Am. J. Trop. Med. Hyg. 81(2) , 356-62, (2009) A randomized, double-blind, placebo-controlled study was conducted to assess the effect of tafenoquine, 200 mg weekly for 6 months on ophthalmic and renal safety. This trial was carried out after observations in previous clinical trials that tafenoquine may b... |
|
|
In-vitro interaction of tafenoquine and chloroquine in Plasmodium falciparum from northwestern Thailand.
Wien. Klin. Wochenschr. 115 Suppl 3 , 28-32, (2003) The blood schizontocidal, pharmacodynamic interaction between tafenoquine (WR 238605--a 5-phenoxyprimaquine derivative--and chloroquine was investigated, using an in-vitro test for the inhibition of schizont maturation, in 15 fresh isolates of Plasmodium falc... |
|
|
Risk factors for gametocyte carriage in uncomplicated falciparum malaria in children before and after artemisinin-based combination treatments.
Chemotherapy 57(6) , 497-504, (2011) Artemisinin-based combination treatments (ACTs) are the recommended first-line antimalarials globally, but their influence on the risk factors associated with gametocyte carriage has had little evaluation in endemic areas.The risk factors associated with game... |
|
|
In vitro activity of tafenoquine against the asexual blood stages of Plasmodium falciparum isolates from Gabon, Senegal, and Djibouti.
Antimicrob. Agents Chemother. 50(9) , 3225-6, (2006)
|
|
|
Tafenoquine: a promising new antimalarial agent.
Expert Opin. Investig. Drugs 16(5) , 705-15, (2007) Malaria remains an important cause of global morbidity and mortality. As antimalarial drug resistance escalates, new safe and effective medications are necessary to prevent and treat malarial infection. Tafenoquine is an 8-aminoquinoline antimalarial that is ... |
|
|
The efficacy and tolerability of three different regimens of tafenoquine versus primaquine for post-exposure prophylaxis of Plasmodium vivax malaria in the Southwest Pacific.
Trans. R. Soc. Trop. Med. Hyg. 102(11) , 1095-101, (2008) Tafenoquine is being developed for radical cure and post-exposure prophylaxis of Plasmodium vivax malaria. In an open-label study, 1512 Australian Defence Force personnel received one of three tafenoquine 3 d regimens [400 mg once daily (od), 200 mg twice dai... |
|
|
Tafenoquine for the treatment of recurrent Plasmodium vivax malaria.
Am. J. Trop. Med. Hyg. 76(3) , 494-6, (2007) Tafenoquine was used to treat Plasmodium vivax malaria cases who had previously failed treatment with chloroquine and primaquine. Chloroquine was followed by a loading dose of tafenoquine (200 mg base/day for 3 days) and 200 mg a week was given for 8 weeks. O... |