Ethyl nipecotate
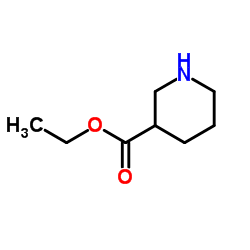
Ethyl nipecotate structure
|
Common Name | Ethyl nipecotate | ||
|---|---|---|---|---|
| CAS Number | 5006-62-2 | Molecular Weight | 157.210 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 238.6±0.0 °C at 760 mmHg | |
| Molecular Formula | C8H15NO2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 90.6±0.0 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Synthesis of stereoisomers of antithrombotic nipecotamides.
Chirality 7 , 90, (1995) The stereoisomers of alpha,alpha'-bis[3-(N,N-diethylcarbamoyl)-piperidino]-p-xylene (1) were synthesized. Rac ethyl nipecotate was resolved by diastereomeric (-)-D- and (+)-L-tartrate salt formation. The enantiomeric esters were hydrolyzed to the correspondin... |
|
|
THIP analgesia: cross tolerance with morphine.
Life Sci. 32(19) , 2265-72, (1983) THIP (4,5,6,7-tetrahydroisoxazolo (5,4-c) pyridone-3-ol), a direct acting GABA receptor agonist, has been shown to have antinociceptive properties. To determine whether tolerance develops to the analgesic response, mice received multiple injections of THIP fo... |
|
|
(R)-nipecotic acid ethyl ester: a direct-acting cholinergic agonist that displays greater efficacy at M2 than at M1 muscarinic receptors.
J. Pharmacol. Exp. Ther. 242(1) , 173-8, (1987) Previous reports have suggested that the ethyl ester of (R)-nipecotic acid ethyl ester [(R)-NAEE] displays cholinomimetic properties in vivo. The present study was undertaken to characterize more fully this action by examining the effects of (R)-NAEE in a num... |
|
|
Differential effects of ethyl (R,S)-nipecotate on the behaviors of highly and minimally aggressive female golden hamsters.
Psychopharmacology 89(4) , 444-8, (1986) The GABA uptake inhibitor ethyl (R,S)-nipecotate produces a dose-dependent suppression of aggression in highly aggressive hamsters but not in minimally aggressive ones. This suppression occurs at doses below those producing peripheral cholinergic effects; at ... |
|
|
Up-regulation of gamma-aminobutyric acid transporter I mediates ethanol sensitivity in mice.
Neuroscience 123(4) , 807-12, (2004) Ethanol is among the most widely abused drugs in the world. Chronic ethanol consumption leads to ethanol tolerance and addiction, and impairs learning and memory. Na+/Cl- dependent GABA transporters play an important role in controlling the concentration of G... |
|
|
Separation of the S(+) and R(-)-enantiomers of tiagabine.HCl and its two chiral precursors by chiral chromatography: application to chiral inversion studies.
J. Pharm. Biomed. Anal. 17(8) , 1439-47, (1998) Chiral HPLC methods were developed and validated for tiagabine.HCl and its two chiral precursors to determine the chiral purity of the three compounds to ensure the quality of the final product which is used as a new antiepileptic drug. Tiagabine.HCl was deri... |
|
|
Increase in drug-induced seizure susceptibility of transgenic mice overexpressing GABA transporter-1.
Acta Pharmacol. Sin. 24(10) , 991-5, (2003) The changes of seizure susceptibility of transgenic mice overexpressing GABA transporter-1 (GAT-1) were studied to clarify the possible role of GABAergic transmission in epileptogenesis.Seizures were induced by intraperitoneal administration of pentylenetetra... |
|
|
Hyperalgesic effects of gamma-aminobutyric acid transporter I in mice.
J. Neurosci. Res. 73(4) , 565-72, (2003) The present study focused on the involvement of gamma-aminobutyric acid transporter I (GAT1) in pain. We found that GABA uptake was increased in mouse spinal cord at 20 min and 120 min after formalin injection and in mouse brain at 120 min, but not 20 min, af... |
|
|
4-(1,2,5,6-Tetrahydro-1-alkyl-3-pyridinyl)-2-thiazolamines: a novel class of compounds with central dopamine agonist properties.
J. Med. Chem. 33(1) , 311-7, (1990) The design, synthesis, and pharmacological properties of a novel type of 4-(1,2,5,6-tetrahydro-1-alkyl-3-pyridinyl)-2-thiazolamine with dopaminergic properties are described. In particular, 4-(1,2,5,6-tetrahydro-1-propyl-3-pyridinyl)-2-thiazolamine (4c, PD 11... |