Calmagite
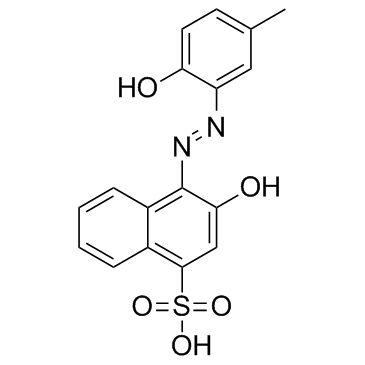
Calmagite structure
|
Common Name | Calmagite | ||
|---|---|---|---|---|
| CAS Number | 3147-14-6 | Molecular Weight | 358.368 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C17H14N2O5S | Melting Point | 300ºC | |
| MSDS | USA | Flash Point | N/A | |
|
Methods for the estimation of serum magnesium in clinical laboratories.
Magnesium 5(5-6) , 317-27, (1986) A variety of methods have been reported for the quantitative analysis of magnesium in serum and other biological fluids. The methods may be classified into the following groups: magnesium ammonium phosphate; 8-hydroxyquinoline; titan yellow; dihydroxyazobenze... |
|
|
Stabilization of the calmagite reagent for automated measurement of magnesium in serum and urine.
Clin. Chem. 31 , 487, (1985)
|
|
|
Clustering of serum calcium and magnesium concentrations in siblings.
Clin. Chem. 32(2) , 349-50, (1986) Concentrations of both serum calcium, adjusted for albumin, and serum magnesium of siblings from 23 families were found to cluster around different concentrations within the normal reference interval. Variation between families accounted for 37% of the total ... |
|
|
Total Mg2+ measured in serum and urine in the Technicon RA 1000 random access analyzer by use of modified manual calmagite procedure.
Clin. Chem. 30(7) , 1262, (1984)
|
|
|
Spectrophotometric determination of acidity constants by two-rank annihilation factor analysis.
Anal. Chim. Acta 607(2) , 142-52, (2008) Annihilation of the contribution of one chemical component from the original data matrix is a general method in rank annihilation factor analysis (RAFA). However, RAFA is not applicable for studying the protonation equilibria of multiprotic acids. In this wor... |
|
|
Avoiding serum matrix effect in calmagite compleximetric measurements of magnesium.
Clin. Chem. 33(5) , 719, (1987)
|
|
|
Micellar improvement of the calmagite compleximetric measurement of magnesium in plasma.
Clin. Chem. 28(3) , 520-2, (1982) Magnesium in plasma is determined by diluting a 50-microL sample with 5.0 mL of stable calmagite reagent containing the amphoteric detergent Empigen BB, and buffered with 2-amino-2-methyl-1-propanol at pH 11.5. The calcium response is masked with strontium-bu... |
|
|
Assay of magnesium in serum and urine with use of only one enzyme, isocitrate dehydrogenase (NADP+).
Clin. Chem. 41(9) , 1302-5, (1995) We report a method for assaying magnesium in serum and urine involving only one enzyme, isocitrate dehydrogenase (NADP+)(EC 1.1.1.42), which requires magnesium ion for activity. The enzymatic reduction of NADP+ by isocitrate increases in rate linearly up to a... |
|
|
Assessment of the functionality of a pilot-scale reactor and its potential for electrochemical degradation of calmagite, a sulfonated azo-dye.
Chemosphere 73(5) , 837-43, (2008) Electrochemical degradation (ECD) is a promising technology for in situ remediation of diversely contaminated environmental matrices by application of a low level electric potential gradient. This investigation, prompted by successful bench-scale ECD of trich... |
|
|
Calmagite dye oxidation using in situ generated hydrogen peroxide catalysed by manganese(II) ions.
Dalton Trans. (44) , 5119-22, (2007) Hydrogen peroxide (H(2)O(2)) generated from the manganese(II) catalysed reduction of dioxygen has been shown to efficiently oxidize Calmagite (3-hydroxy-4-(2-hydroxy-5-methylphenylazo)naphthalene-1-sulfonic acid) in aqueous solution at pH 8.0 and 20 +/- 1 deg... |