Assessment of the functionality of a pilot-scale reactor and its potential for electrochemical degradation of calmagite, a sulfonated azo-dye.
Shirish Agarwal, Phillip Cluxton, Mark Kemper, Dionysios D Dionysiou, Souhail R Al-Abed
Index: Chemosphere 73(5) , 837-43, (2008)
Full Text: HTML
Abstract
Electrochemical degradation (ECD) is a promising technology for in situ remediation of diversely contaminated environmental matrices by application of a low level electric potential gradient. This investigation, prompted by successful bench-scale ECD of trichloroethylene, involved development, parametric characterization and evaluation of a pilot-scale electrochemical reactor for degradation of calmagite, a sulfonated azo-dye used as a model contaminant. The reactor has two chambers filled with granulated graphite for electrodes. The system has electrical potential, current, conductivity, pH, temperature, water-level and flow sensors for automated monitoring. The reactor supports outdoor and fail-safe venting, argon purging, temperature regulation and auto-shutdown for safety. Treatment involves recirculating the contaminated solution through the electrode beds at small flow velocities mimicking low fluid-flux in groundwater and submarine sediments. The first phase of the investigation involved testing of the reactor components, its parametric probes and the automated data acquisition system for performance as designed. The results showed hydraulic stability, consistent pH behavior, marginal temperature rise (<5 degrees C) and overall safe and predictable performance under diverse conditions. Near complete removal of calmagite was seen at 3-10V of applied voltage in 8-10h. The effects of voltage and strength of electrolyte on degradation kinetics have been presented. Further, it was observed from the absorption spectra that as calmagite degrades over time, new peaks appear. These peaks were associated with degradation products identified using electrospray ionization mass spectrometry. A reaction mechanism for ECD of calmagite has also been proposed.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
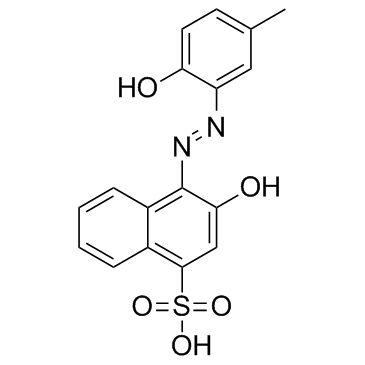 |
Calmagite
CAS:3147-14-6 |
C17H14N2O5S |
|
Methods for the estimation of serum magnesium in clinical la...
1986-01-01 [Magnesium 5(5-6) , 317-27, (1986)] |
|
Stabilization of the calmagite reagent for automated measure...
1985-03-01 [Clin. Chem. 31 , 487, (1985)] |
|
Clustering of serum calcium and magnesium concentrations in ...
1986-02-01 [Clin. Chem. 32(2) , 349-50, (1986)] |
|
Total Mg2+ measured in serum and urine in the Technicon RA 1...
1984-07-01 [Clin. Chem. 30(7) , 1262, (1984)] |
|
Spectrophotometric determination of acidity constants by two...
2008-01-28 [Anal. Chim. Acta 607(2) , 142-52, (2008)] |