(3-beta,5-beta,6-beta)-5,6-Epoxycholestan-3-ol
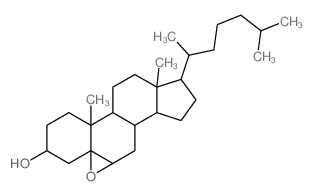
(3-beta,5-beta,6-beta)-5,6-Epoxycholestan-3-ol structure
|
Common Name | (3-beta,5-beta,6-beta)-5,6-Epoxycholestan-3-ol | ||
|---|---|---|---|---|
| CAS Number | 4025-59-6 | Molecular Weight | 402.65300 | |
| Density | 1.04g/cm3 | Boiling Point | 497.7ºC at 760 mmHg | |
| Molecular Formula | C27H46O2 | Melting Point | N/A | |
| MSDS | USA | Flash Point | 200.9ºC | |
|
Surprising unreactivity of cholesterol-5,6-epoxides towards nucleophiles.
J. Lipid Res. 53(4) , 718-25, (2012) We recently established that drugs used for the treatment and the prophylaxis of breast cancers, such as tamoxifen, were potent inhibitors of cholesterol-5,6-epoxide hydrolase (ChEH), which led to the accumulation of 5,6α-epoxy-cholesterol (5,6α-EC) and 5,6β-... |
|
|
Formation of biologically active oxysterols during ozonolysis of cholesterol present in lung surfactant.
J. Biol. Chem. 279(25) , 26331-8, (2004) Exposure of the lung to concentrations of ozone found in ambient air is known to cause toxicity to the epithelial cells of the lung. Because of the chemical reactivity of ozone, it likely reacts with target molecules in pulmonary surfactant, a lipid-rich mate... |
|
|
Comparative study of the cytotoxicity and apoptosis-inducing potential of commonly occurring oxysterols.
Cell Biol. Toxicol. 17(2) , 127-37, (2001) The cytotoxicity of the oxysterols 25-hydroxycholesterol, 7beta-hydroxycholesterol, cholesterol-5alpha,6alphaepoxide, cholesterol-5beta,6beta-epoxide, 19-hydroxycholesterol and 7-ketocholesterol was examined in U937 cells, a human monocytic blood cell line. 7... |
|
|
The role of the mitochondria in apoptosis induced by 7beta-hydroxycholesterol and cholesterol-5beta,6beta-epoxide.
Br. J. Nutr. 94(4) , 519-25, (2005) Oxysterols are oxygenated derivatives of cholesterol that may be formed endogenously or absorbed from the diet. Significant amounts of oxysterols have frequently been identified in foods of animal origin, in particular highly processed foods. To date, oxyster... |
|
|
An excess concentration of oxysterols in the plasma is cytotoxic to cultured endothelial cells.
Atherosclerosis 149(1) , 191-7, (2000) To test if there is an excess concentration of oxysterols in the plasma of the patients with cardiovascular disease, we analyzed the oxysterol content in the plasma from 105 cardiac catheterized patients with angina and 80+/-8% stenosis in their coronary arte... |
|
|
Inhibition by cholesterol oxides of NO release from human vascular endothelial cells.
Arterioscler. Thromb. Vasc. Biol. 18(7) , 1054-60, (1998) Recent studies have demonstrated that, unlike cholesterol, cholesterol oxidized at position 7 can reduce the maximal endothelium-dependent relaxation of isolated rabbit aortas (Circulation. 1997;95:723-731). The aim of the current study was to determine wheth... |
|
|
Oxidized cholesterol in the diet is a source of oxidized lipoproteins in human serum.
J. Lipid Res. 44(4) , 705-15, (2003) The aim of this study was to determine in humans whether oxidized cholesterol in the diet is absorbed and contributes to the pool of oxidized lipids in circulating lipoproteins. When a meal containing 400 mg cholestan-5alpha,6alpha-epoxy-3beta-ol (alpha-epoxy... |
|
|
Cholesterol-5,6-epoxides: chemistry, biochemistry, metabolic fate and cancer.
Biochimie 95(3) , 622-31, (2013) In the nineteen sixties it was proposed that cholesterol might be involved in the etiology of cancers and cholesterol oxidation products were suspected of being causative agents. Researchers had focused their attention on cholesterol-5,6-epoxides (5,6-ECs) ba... |
|
|
Comparison of the apoptotic processes induced by the oxysterols 7beta-hydroxycholesterol and cholesterol-5beta,6beta-epoxide.
Cell Biol. Toxicol. 20(5) , 313-23, (2004) Oxysterols have been shown to induce apoptosis in a variety of cell lines. The mechanism of oxysterol-induced apoptosis is mainly known at the post-mitochondrial level. The aim of the present study was to compare the pathway of apoptosis induced by the oxyste... |
|
|
Role of epoxide hydrolases in lipid metabolism.
Biochimie 95(1) , 91-5, (2013) Epoxide hydrolases (EH), enzymes present in all living organisms, transform epoxide-containing lipids to 1,2-diols by the addition of a molecule of water. Many of these oxygenated lipid substrates have potent biological activities: host defense, control of de... |