Seneciphylline
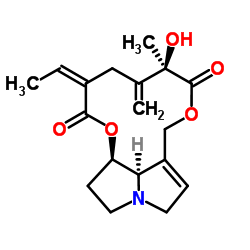
Seneciphylline structure
|
Common Name | Seneciphylline | ||
|---|---|---|---|---|
| CAS Number | 480-81-9 | Molecular Weight | 333.379 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 577.7±50.0 °C at 760 mmHg | |
| Molecular Formula | C18H23NO5 | Melting Point | 217ºC | |
| MSDS | Chinese USA | Flash Point | 303.2±30.1 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
|
Interplant alkaloid variation and Senecio vernalis toxicity in cattle.
Vet. Hum. Toxicol. 43(3) , 147-51, (2001) Senecio vernalis and other plants containing pyrrolizidine alkaloids (PA) are implicated in the poisoning of cattle. The liver is a known target organ. In this study the content of the alkaloids senecionine (SCO), senkirkin (SKK) and seneciphyllin (SCP) and t... |
|
|
Pyrrolizidine alkaloids in pollen and pollen products.
Mol. Nutr. Food. Res. 54(2) , 292-300, (2010) Recently, 1,2-dehydropyrrolizidine alkaloid (PA) ester alkaloids, found predominantly as their N-oxides (PANOs, pyrrolizidine N-oxides), have been reported in both honey and in pollen obtained directly from PA plants and pollen loads collected by bees, raisin... |
|
|
Mutagenic activity of the pyrrolizidine alkaloids seneciphylline and senkirkine in Drosophila and their transfer into rat milk.
Food Chem. Toxicol. 22(3) , 223-5, (1984) Seneciphylline and senkirkine, two pyrrolizidine alkaloids that occur in animal feeds and medicinal herbs, respectively, have been tested for their ability to produce sex-linked recessive lethals in males of Drosophila melanogaster using the Basc test (3-day ... |
|
|
Pyrrolizidine alkaloids from Senecio leucanthemifolius and Senecio rodriguezii.
Nat. Toxins 1(4) , 241-5, (1993) Two toxic pyrrolizidine alkaloids were isolated from Senecio leucanthemifolius and six from Senecio rodriguezii. Their structures were determined by spectroscopic methods. |
|
|
Microsomal metabolism of pyrrolizidine alkaloids: N-oxidation of seneciphylline and senecionine.
Toxicol. Lett. 37(3) , 241-9, (1987) In vivo pretreatment of rats with phenobarbital or beta-naphthoflavone reduced the specific activity of microsomal pyrrolizidine alkaloid N-oxide formation. Heat pretreatment of microsomes under conditions intended to selectively inactivate the flavin-contain... |
|
|
Effect of seneciphylline and senecionine on hepatic drug metabolizing enzymes in rats.
J. Ethnopharmacol. 12(3) , 271-8, (1984) The effect of oral administration of the pyrrolizidine alkaloids, seneciphylline and senecionine, from Senecio vulgaris (Compositae) on activities of hepatic epoxide hydrase, glutathione-S-transferase, aminopyrine-N-demethylase and arylhydrocarbon hydroxylase... |
|
|
Transfer of 14C-seneciphylline into sheep milk following multiple oral intakes.
DTW Dtsch. Tierarztl. Wochenschr. 104(3) , 97-8, (1997) Pyrrolizidine alkaloids (PAs) contamination of animal products, may pose a threat to human health. Among ruminants, sheep appear to be more resistant to PAs poisoning and consequently comparatively higher concentrations of the PAs into their milk might be exp... |
|
|
Transfer of [3H]pyrrolizidine alkaloids from Senecio vulgaris L. and metabolites into rat milk and tissues.
Toxicol. Lett. 17(3-4) , 283-8, (1983) [3H]Retronecine and [3H]necic acid-labelled senecionine and seneciphylline were prepared biosynthetically with seedlings of Senecio vulgaris L. using [2,3-3H]putrescine and [4,5-3H]isoleucine, respectively, as precursors. Lactating rats dosed with these diffe... |
|
|
Influence of inhibition of the metabolic activation on the mutagenicity of some nitrosamines, triazenes, hydrazines and seniciphylline in Drosophila melanogaster.
Mutat. Res. 202(1) , 251-67, (1988) It is determined to what extent certain inhibitors of the xenobiotic metabolizing enzyme systems have an influence on the mutagenicity of various pro-mutagens in Drosophila. 1-Phenylimidazole (PhI) is used as an inhibitor of the cytochrome P-450 (P-450) media... |
|
|
In vivo covalent binding of retronecine-labelled [3H]seneciphylline and [3H]senecionine to DNA of rat liver, lung and kidney.
Chem. Biol. Interact. 54(1) , 57-69, (1985) Retronecine-labelled [3H]seneciphylline ([3H]SPH) and [3H]senecionine ([3H]SON) of high specific radioactivity (22 and 49 mCi/mmol, respectively) were prepared biosynthetically with seedlings of Senecio vulgaris L. using [2,3-3H]putrescine as precursor. [2,3-... |