tris(1,1,1,3,3,3-hexafluoro-2-propyl) phosphite
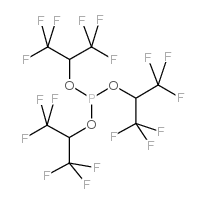
tris(1,1,1,3,3,3-hexafluoro-2-propyl) phosphite structure
|
Common Name | tris(1,1,1,3,3,3-hexafluoro-2-propyl) phosphite | ||
|---|---|---|---|---|
| CAS Number | 66470-81-3 | Molecular Weight | 532.06300 | |
| Density | 1.69 g/mL at 25ºC(lit.) | Boiling Point | 130ºC(lit.) | |
| Molecular Formula | C9H3F18O3P | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 44.5ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Solid phase synthesis of oligoribonucleotides using the 1-[(2-chloro-4-methyl)phenyl]-4-methoxypiperidin-4-yl (Ctmp) group for the protection of the 2'-hydroxy functions and the H-phosphonate approach.
Nucleic Acids Res. 17(10) , 3689-97, (1989) The solid phase synthesis of oligoribonucleotides using the H-phosphonate approach and the 1-[(2-chloro-4-methyl)phenyl]-4-methoxypiperidin-4-yl (Ctmp) and dimethoxytrityl (DMTr) groups, respectively, for the protection of the 2'- and 5'-hydroxy functions is ... |
|
|
Use of new phosphonylating and coupling agents in the synthesis of oligodeoxyribonucleotides via the H-phosphonate approach.
Nucleic Acids Res. 18(11) , 3327-31, (1990) New phosphonylating and coupling agents for the synthesis of oligodeoxyribonucleotides via H-phosphonate approach have been developed. Tris(1,1,1,3,3,3-hexafluoro-2-propyl) phosphite, prepared by the reaction of lithium salt of 1,1,1,3,3,3-hexafluoro-2-propox... |
|
|
Stereoselective propargylation mediated by a chiral metal cluster: reactions of [(propargylium)Co2 (CO)5{P(OR)3}][BF4] with carbon nucleophiles. Caffyn AJM and Nicholas KM.
J. Am. Chem. Soc. 115(14) , 6438-6439, (1993)
|
|
|
Synthesis of antisense oligodeoxyribonucleotide analogues by use of deoxyribonucleoside 3'-bis(1,1, 1,3,3,3-hexafluoro-2-propyl)phosphites as new key intermediates. Hosaka H, et al.
Heteroatom Chem. 2(1) , 197-204, (1991)
|
|
|
Preparation and nuclear magnetic resonance studies of pentacoordinated phosphorus compounds containing hexafluoroisopropoxy groups. Denney DB, et al.
J. Org. Chem. 48(13) , 2159-2164, (1983)
|