5-Bromonicotinic acid
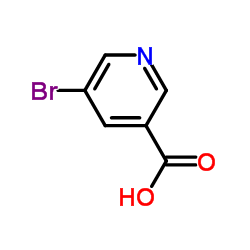
5-Bromonicotinic acid structure
|
Common Name | 5-Bromonicotinic acid | ||
|---|---|---|---|---|
| CAS Number | 20826-04-4 | Molecular Weight | 202.01 | |
| Density | 1.8±0.1 g/cm3 | Boiling Point | 328.5±27.0 °C at 760 mmHg | |
| Molecular Formula | C6H4BrNO2 | Melting Point | 178-180 °C(lit.) | |
| MSDS | USA | Flash Point | 152.5±23.7 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Agonist lead identification for the high affinity niacin receptor GPR109a.
Bioorg. Med. Chem. Lett. 17 , 4914-9, (2007) A strategy for lead identification of new agonists of GPR109a, starting from known compounds shown to activate the receptor, is described. Early compound triage led to the formulation of a binding hypothesis and eventually to our focus on a series of pyrazole... |
|
|
Iodopyridine-for-iodobenzene substitution for use with low molecular weight radiopharmaceuticals: application to m-iodobenzylguanidine.
Bioconjug. Chem. 9(6) , 758-64, (1998) Substituting a pyridine ring for a benzene ring in the acylation agent N-succinimidyl 3-iodobenzoate has resulted in a useful approach for the radiohalogenation of monoclonal antibodies, peptides, and labeled biotin conjugates. It was hypothesized that such a... |
|
|
Drug-protein conjugates: haptenation of 1-methyl-10 alpha-methoxydihydrolysergol and 5-bromonicotinic acid to albumin for the production of epitope-specific monoclonal antibodies against nicergoline.
J. Pharm. Sci. 84(9) , 1120-5, (1995) Two types of monoclonal antibodies were used for the determination of nicergoline in biological matrices. The antibodies were prepared with the hydrolysis products 5-bromonicotinic acid and 1-methyl-10 alpha-methoxydihydrolysergol after hemisuccinoylation to ... |
|
|
Functional diversity among 5-substituted nicotine analogs; in vitro and in vivo investigations.
Eur. J. Pharmacol. 435(2-3) , 171-80, (2002) Two 5-substituted derivatives of nicotine (nicotinic acetylcholine receptor: K(i)=2.4 nM) were synthesized and evaluated: 5-bromonicotine (K(i)=6.9 nM) and 5-methoxynicotine (K(i)=14.3 nM). Despite their high affinity, neither 5-bromonicotine nor 5-methoxynic... |