Triprolidine hydrochloride
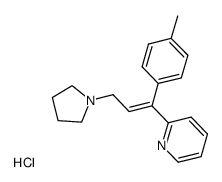
Triprolidine hydrochloride structure
|
Common Name | Triprolidine hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 6138-79-0 | Molecular Weight | 314.85200 | |
| Density | N/A | Boiling Point | 462ºC at 760 mmHg | |
| Molecular Formula | C19H25ClN2O | Melting Point | 115-120ºC | |
| MSDS | Chinese USA | Flash Point | 233.2ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Involvement of histaminergic inputs in the jaw-closing reflex arc.
J. Neurophysiol. 113 , 3720-35, (2015) Histamine receptors are densely expressed in the mesencephalic trigeminal nucleus (MesV) and trigeminal motor nucleus. However, little is known about the functional roles of neuronal histamine in controlling oral-motor activity. Thus, using the whole-cell rec... |
|
|
Development and validation of an ultra-performance liquid chromatography method for simultaneous analysis of 20 antihistaminics in dietary supplements.
Biomed. Chromatogr. 29(3) , 465-74, (2015) The purpose of this study was to develop and validate an ultra-performance liquid chromatography method for simultaneous analysis of 20 antihistamines (illegal additives) in dietary supplements. The limits of detection and quantitation of the method ranged fr... |
|
|
Quantification of antihistamine acrivastine in plasma by solid-phase extraction and high-performance liquid chromatography.
J. Pharm. Biomed. Anal. 43(1) , 293-7, (2007) An automated solid-phase extraction method was developed for the determination of the H1-antihistamine acrivastine in plasma samples. Acrivastine was analyzed at the wavelength of 254 nm using a reversed-phase HPLC assay. Both extraction procedure and analyti... |
|
|
Spectrophotometric determination of some antihistaminic drugs using 7,7,8,8-tetracyanoquinodimethane (TCNQ).
J. AOAC Int. 89(1) , 46-52, (2006) A simple and sensitive spectrophotometric method is suggested for analysis of 3 antihistaminic drugs, acrivastine (I), mequitazine (II), and dimethindene maleate (III). The method is based on reaction of the drugs with 7,7,8,8-tetracyanoquinodimethane (TCNQ) ... |
|
|
Development and validation of a liquid chromatography-tandem mass spectrometry method for the simultaneous determination of acrivastine and pseudoephedrine in human plasma and its application in pharmacokinetics.
Arzneimittelforschung 62(10) , 449-56, (2012) A specific, sensitive and accurate liquid chromatography-tandem mass spectrometry (LC-MS/MS) method has been developed for the simultaneous determination of acrivastine and pseudoephedrine in human plasma samples. Plasma samples were processed and analyzed on... |
|
|
Synthesis of aryl(di)azinyl ketones through copper- and iron-catalyzed oxidation of the methylene group of aryl(di)azinylmethanes.
Angew. Chem. Int. Ed. Engl. 51(11) , 2745-8, (2012) Sustainable Oxidations: an oxidation method to transform aryl(di)azinylmethanes into aryl(di)azinyl ketones is described. Base metals (copper and iron) as catalysts in combination with O(2) as the oxidant are used, which makes this method sustainable. The uti... |
|
|
The butterbur extract petasin has no effect on skin test reactivity induced by different stimuli: a randomized, double-blind crossover study using histamine, codeine, methacholine, and aeroallergen solutions.
J. Investig. Allergol. Clin. Immunol. 16(3) , 156-61, (2006) Petasin (Ze 339) was recently introduced on the market as a potent herbal antiallergic drug for treatment of respiratory allergies such as hay fever. Few clinical studies have been performed so far addressing the clinical effectiveness of Ze 339.To evaluate t... |
|
|
["Of snakes and crocodiles": central side effects of nose drops and facts about rhinitis medicamentosa].
Kinderkrankenschwester 30(10) , 406-8, (2011)
|
|
|
Endogenous histamine facilitates long-term potentiation in the hippocampus during walking.
J. Neurosci. 30 , 7845-52, (2010) Long-term potentiation (LTP) in hippocampal CA1 depends on the behavioral state of LTP induction. We hypothesize that histaminergic activity in the septohippocampal system, which is active during walking compared with other behavioral states, is responsible f... |
|
|
Dopamine transporter-dependent and -independent striatal binding of the benztropine analog JHW 007, a cocaine antagonist with low abuse liability.
J. Pharmacol. Exp. Ther. 335(3) , 703-14, (2010) The benztropine analog N-(n-butyl)-3α-[bis(4'-fluorophenyl)methoxy]-tropane (JHW 007) displays high affinity for the dopamine transporter (DAT), but unlike typical DAT ligands, has relatively low abuse liability and blocks the effects of cocaine, including it... |