1-Brom-3,3-dimethylbutan-2-on
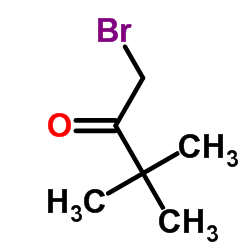
1-Brom-3,3-dimethylbutan-2-on structure
|
Common Name | 1-Brom-3,3-dimethylbutan-2-on | ||
|---|---|---|---|---|
| CAS Number | 5469-26-1 | Molecular Weight | 179.055 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 189.5±0.0 °C at 760 mmHg | |
| Molecular Formula | C6H11BrO | Melting Point | −10 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 43.8±7.2 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
SmI2-promoted Reformatsky-type coupling reactions in exceptionally hindered contexts.
Org. Lett. 10(6) , 1291-4, (2008) Highly substituted, very hindered enones were synthesized using a two-step procedure that utilizes a diiodosamarium-promoted Reformatsky-type coupling and dehydration using Martin sulfurane. Both alpha-chloro- and alpha-bromoketones were coupled with a variet... |
|
|
Synthesis and biological activity of an azido derivative of paclobutrazol, an inhibitor of gibberellin biosynthesis.
Plant Physiol. 88(4) , 1425-9, (1988) A photolabile azido derivative of the kaurene oxidase inhibitor 1-(4-chlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-l-yl) pentan-3-ol (paclobutrazol) has been synthesized for use as a photoaffinity labeling agent. The compound was tested as an inhibitor of the o... |
|
|
1-Bromopinacolone, an active site-directed covalent inhibitor for acetylcholinesterase.
J. Biol. Chem. 257(23) , 14087-92, (1982) 1-Bromopinacolone, BrPin, acts initially as a reversible competitive inhibitor for acetylcholinesterase, KI = 0.18 mM in hydrolysis of acetylcholine. Unlike bromoacetone, with time it acts as an irreversible covalent inhibitor. BrPin has a hydrolytic half-lif... |
|
|
Reactions of 1-bromo-2-[14C]pinacolone with acetylcholinesterase from Torpedo nobiliana. Effects of 5-trimethylammonio-2-pentanone and diisopropyl fluorophosphate.
Biochim. Biophys. Acta 997(3) , 167-75, (1989) 1-Bromo-2-[14C]pinacolone, (CH3)3C14COCH2Br [( 14C]BrPin), was prepared from [1-14C]acetyl chloride and tert-butylmagnesium chloride with cuprous chloride catalyst, followed by bromination. It was examined as an active-site directed label for acetylcholineste... |
|
|
Active-site peptides of acetylcholinesterase of Electrophorus electricus: labelling of His-440 by 1-bromo-[2-14C]pinacolone and Ser-200 by tritiated diisopropyl fluorophosphate.
Biochim. Biophys. Acta 1208(2) , 324-31, (1994) To characterize the structure of the active site of acetylcholinesterase (AChE) from the electric organ of E. electricus, we identified sites of incorporation of two active-site affinity labels, [3H]diisopropyl fluorophosphate ([3H]DFP), and 1-bromo-2-[14C]pi... |
|
|
Labeling of cysteine 231 in acetylcholinesterase from Torpedo nobiliana by the active-site directed reagent, 1-bromo-2-[14C] pinacolone. Effects of 2,2'-dipyridyl disulfide and other sulfhydryl reagents.
J. Biol. Chem. 268(1) , 245-51, (1993) Acetylcholinesterase (AcChE, EC 3.1.1.7) was isolated from the electric organ of T. nobiliana and treated with the active-site-directed alkylating agent 1-bromo-2-[14C]pinacolone ([14C]BrPin), or with BrPin, which acts initially as a competitive inhibitor, Ki... |
|
|
Methyl imidazo [1,2-b] pyridazine-2-carbamates and related compounds as potential antifilarial agents Mourad AE, et al.
J. Heterocycl. Chem. 29 , 1583, (1992)
|