4-Tert-Butylpyridine
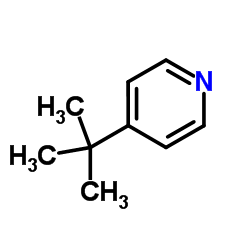
4-Tert-Butylpyridine structure
|
Common Name | 4-Tert-Butylpyridine | ||
|---|---|---|---|---|
| CAS Number | 3978-81-2 | Molecular Weight | 135.206 | |
| Density | 0.923 | Boiling Point | 196-197 ºC | |
| Molecular Formula | C9H13N | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 67 ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Fabrication of Mesoporous CoS2 Nanotube Arrays as the Counter Electrodes of Dye-Sensitized Solar Cells.
Chem. Asian J. 10 , 1932-9, (2015) Mesoporous cobalt sulfide nanotube arrays on FTO-coated glass were synthesized by combining three simple technologies: the selective etching of ZnO sacrificial templates, mesoporous Co3 O4 formation from cobalt-chelated chitosan, and ion-exchange reaction (IE... |
|
|
In situ Poly(methyl methacrylate)/Graphene Composite Gel Electrolytes for Highly Stable Dye-Sensitized Solar Cells.
ChemSusChem 8 , 3799-804, (2015) Dye-sensitized solar cells (DSCs) with long-term stability are produced using polymer-gel electrolytes (PGEs). In this study, we introduce the formation of PGEs using in situ gelation with poly(methyl methacrylate) (PMMA) particles and graphene fillers that a... |
|
|
Dispelling clichés at the nanoscale: the true effect of polymer electrolytes on the performance of dye-sensitized solar cells.
Nanoscale 7 , 12010-7, (2015) In the field of dye-sensitized solar cells, polymer electrolytes are among the most studied materials due to their ability to ensure both high efficiency and stability, the latter being a critical point of these devices. Hundreds of polymeric matrices have be... |
|
|
A simple route to making counter electrode for dye sensitized solar cells (DSSCs) using sucrose as carbon precursor.
J. Colloid. Interface Sci. 459 , 146-50, (2015) Dye sensitized solar cells (DSSCs) have attracted much attention in recent years due to low cost fabrication as compared to silicon-based and thin film solar cells. Though, platinum is an excellent catalytic material for use in preparation of counter electrod... |
|
|
Control of I-V hysteresis in CH3NH3PbI3 perovskite solar cell.
J. Phys. Chem. Lett. 6 , 4633-9, (2015) Mismatch of current (I)-voltage (V) curves with respect to the scan direction, so-called I-V hysteresis, raises critical issue in MAPbI3 (MA = CH3NH3) perovskite solar cell. Although ferroelectric and ion migration have been proposed as a basis for the hyster... |
|
|
Multifunctional MgO Layer in Perovskite Solar Cells.
ChemPhysChem 16 , 1727-32, (2015) A multifunctional magnesium oxide (MgO) layer was successfully introduced into perovskite solar cells (PSCs) to enhance their performance. MgO was coated onto the surface of mesoporous TiO(2) by the decomposition of magnesium acetate and, therefore, could blo... |
|
|
Open circuit potential build-up in perovskite solar cells from dark conditions to 1 sun.
J. Phys. Chem. Lett. 6 , 4640-5, (2015) The high open-circuit potential (Voc) achieved by perovskite solar cells (PSCs) is one of the keys to their success. The Voc analysis is essential to understand their working mechanisms. A large number of CH3NH3PbI3-xClx PSCs were fabricated on single large-a... |
|
|
Hierarchically Structured Hole Transport Layers of Spiro-OMeTAD and Multiwalled Carbon Nanotubes for Perovskite Solar Cells.
ChemSusChem 8 , 2358-62, (2015) The low electrical conductivity of spiro-OMeTAD hole transport layers impedes further enhancements of the power conversion efficiency (PCE) of perovskite solar cells. We embedded multiwalled carbon nanotubes (MWNTs) in spiro-OMeTAD (spiro-OMeTAD/MWNTs) to inc... |
|
|
On the Role of Interfaces in Planar-Structured HC(NH2 )2 PbI3 Perovskite Solar Cells.
ChemSusChem 8 , 2414-9, (2015) Planar-structured HC(NH2 )2 PbI3 (FAPbI3 ) perovskite solar cells were prepared via a two-step deposition process. To investigate the role of interface, the perovskite morphology was intentionally modified by varying HC(NH2 )2 I concentration. Surface and gra... |
|
|
High-performance electrocatalysis using metallic cobalt pyrite (CoS₂) micro- and nanostructures.
J. Am. Chem. Soc. 136(28) , 10053-61, (2014) The development of efficient and robust earth-abundant electrocatalysts for the hydrogen evolution reaction (HER) is an ongoing challenge. We report metallic cobalt pyrite (cobalt disulfide, CoS2) as one such high-activity candidate material and demonstrate t... |