Adamantane-1-carboxylic acid
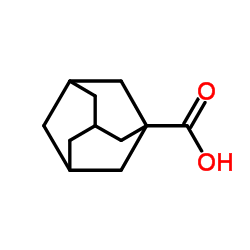
Adamantane-1-carboxylic acid structure
|
Common Name | Adamantane-1-carboxylic acid | ||
|---|---|---|---|---|
| CAS Number | 828-51-3 | Molecular Weight | 180.243 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 304.7±10.0 °C at 760 mmHg | |
| Molecular Formula | C11H16O2 | Melting Point | 172-174 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 142.0±13.7 °C | |
|
Novel Bradykinin Analogues Modified in the N-Terminal Part of the Molecule with a Variety of Acyl Substituents.
Int. J. Pept. Res. Ther. 18(2) , 117-124, (2012) In the current work we present some pharmacological characteristics of ten new analogues of bradykinin (Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg) modified in the N-terminal part of the molecule with a variety of acyl substituents. Of the many acylating agents used... |
|
|
Trace analysis of total naphthenic acids in aqueous environmental matrices by liquid chromatography/mass spectrometry-quadrupole time of flight mass spectrometry direct injection.
J. Chromatogr. A. 1405 , 49-71, (2015) A rapid and sensitive liquid chromatography quadrupole time of flight method has been established for the determination of total naphthenic acid concentrations in aqueous samples. This is the first methodology that has been adopted for routine, high resolutio... |
|
|
Unexpected fluorescent behavior of a 4-amino-1,8-naphthalimide derived beta-cyclodextrin: conformation analysis and sensing properties.
Chem. Commun. (Camb.) (27) , 4091-3, (2009) A new fluorescent cyclodextrin shows unexpected and strong fluorescence enhancement upon binding of organic molecules, and the enhancing mechanism is found to be different from those reported in the literature. |
|
|
Solutes probe hydration in specific association of cyclodextrin and adamantane.
J. Am. Chem. Soc. 127(7) , 2184-90, (2005) Using microcalorimetry, we follow changes in the association free energy of beta-cyclodextrin (CD) with the hydrophobic part of adamantane carboxylate (AD) due to added salt or polar (net-neutral) solutes that are excluded from the molecular interacting surfa... |
|
|
1H NMR studies on the hydrogen-bonding network in mono-altro-beta-cyclodextrin and its complex with adamantane-1-carboxylic acid.
Carbohydr. Res. 340(8) , 1539-45, (2005) The hydrogen-bond network in mono-altro-beta-cyclodextrin and in its inclusion complex with adamantane-1-carboxylic acid were investigated by (1)H NMR spectroscopy using the chemical shifts, temperature coefficients and vicinal coupling constants of the excha... |
|
|
Quinolinotriazole-beta-cyclodextrin and its adamantanecarboxylic acid complex as efficient water-soluble fluorescent Cd(2+) sensors.
Bioorg. Med. Chem. 18(4) , 1415-20, (2010) A novel beta-cyclodextrin derivative 1 bearing 8-hydroxyquinolino and triazole groups was synthesized in satisfactory yield by 'click chemistry'. With a good water solubility up to 0.03 mol/L, 1 exhibited an effective switch-on fluorescence response to Cd(2+)... |
|
|
Artificial chaperone-assisted refolding of denatured-reduced lysozyme: modulation of the competition between renaturation and aggregation.
Biochemistry 35(49) , 15760-71, (1996) Conditions that promote renaturation of an unfolded protein also promote protein aggregation, in many cases, because these competing intramolecular and intermolecular processes are driven by similar networks of noncovalent interactions. The GroEL/GroES system... |
|
|
Electrorotation--a new method for investigating membrane events during thrombocyte activation. Influence of drugs and osmotic pressure.
Biochim. Biophys. Acta 861(1) , 122-30, (1986) The measurement of the spin of cells in rotating high-frequency electric fields ('electrorotation') make possible the investigation of dielectric membrane properties of single cells. This method was applied to membrane permeability changes accompanying thromb... |
|
|
[New adamantane derivatives and ways of overcoming the resistance of influenza A viruses to rimantadine and amantadine].
Vopr. Virusol. 56(2) , 36-9, (2011) The amino acid and peptide derivatives of 1-adamantane carboxylic acid and rimantadine (18 compounds) have been first synthesized and investigated for their activity against influenza A virus (H1N1, H1N1v). In a series of obtained adamantine derivatives, some... |
|
|
Synthesis of porous platinum nanoparticles.
Small 2(2) , 249-53, (2006)
|