1H NMR studies on the hydrogen-bonding network in mono-altro-beta-cyclodextrin and its complex with adamantane-1-carboxylic acid.
Birgit Hakkarainen, Kahee Fujita, Stefan Immel, Lennart Kenne, Corine Sandström
Index: Carbohydr. Res. 340(8) , 1539-45, (2005)
Full Text: HTML
Abstract
The hydrogen-bond network in mono-altro-beta-cyclodextrin and in its inclusion complex with adamantane-1-carboxylic acid were investigated by (1)H NMR spectroscopy using the chemical shifts, temperature coefficients and vicinal coupling constants of the exchangeable hydroxy protons. The chemical shifts of the 3-OH signals indicated that the hydrogen-bond network between the 2-OH and 3-OH groups was disturbed not only on each side of the altrose residue, but also along the whole dextrin chain. Upon addition of adamantane-1-carboxylic acid, altrose underwent a conformational change from the (1)C(4) to the (O)S(2) form, allowing a more continuous belt of hydrogen bonding, as evidenced by the downfield shift experienced by the 3-OH proton signals of the glucose residues.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
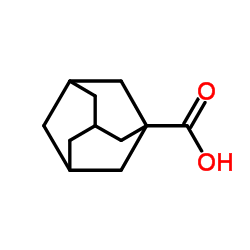 |
Adamantane-1-carboxylic acid
CAS:828-51-3 |
C11H16O2 |
|
Novel Bradykinin Analogues Modified in the N-Terminal Part o...
2012-05-01 [Int. J. Pept. Res. Ther. 18(2) , 117-124, (2012)] |
|
Trace analysis of total naphthenic acids in aqueous environm...
2015-07-31 [J. Chromatogr. A. 1405 , 49-71, (2015)] |
|
Unexpected fluorescent behavior of a 4-amino-1,8-naphthalimi...
2009-07-21 [Chem. Commun. (Camb.) (27) , 4091-3, (2009)] |
|
Solutes probe hydration in specific association of cyclodext...
2005-02-23 [J. Am. Chem. Soc. 127(7) , 2184-90, (2005)] |
|
Quinolinotriazole-beta-cyclodextrin and its adamantanecarbox...
2010-02-15 [Bioorg. Med. Chem. 18(4) , 1415-20, (2010)] |