2,4-Lutidine
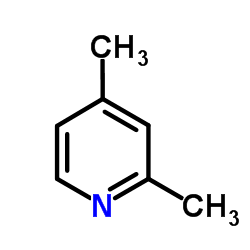
2,4-Lutidine structure
|
Common Name | 2,4-Lutidine | ||
|---|---|---|---|---|
| CAS Number | 108-47-4 | Molecular Weight | 107.153 | |
| Density | 0.9±0.1 g/cm3 | Boiling Point | 157.4±9.0 °C at 760 mmHg | |
| Molecular Formula | C7H9N | Melting Point | -60 °C | |
| MSDS | Chinese USA | Flash Point | 37.2±0.0 °C | |
| Symbol |


GHS02, GHS06 |
Signal Word | Danger | |
|
[Plasmids for biodegradation of 2,6-dimethylpyridine, 2,4-dimethylpyridine, and pyridine in strains of Arthrobacter].
Mol. Gen. Mikrobiol. Virusol. (5-6) , 10-3, (1992) Arthrobacter crysallopoietes strain KM-4 degrading 2,6-dimethylpyridine and strain KM-4a degrading both 2,6-dimethylpyridine and pyridine, Arthrobacter sp. KM-4b degrading 2,4-dimethylpyridine were isolated from soil. Arthrobacter crystallopoietes KM-4 and Ar... |
|
|
Chemical standards in ion mobility spectrometry.
Analyst 135(6) , 1433-42, (2010) In ion mobility spectrometry (IMS), reduced mobility values (K(0)) are used as a qualitative measure of gas phase ions, and are reported in the literature as absolute values. Unfortunately, these values do not always match with those collected in the field. O... |
|
|
Factors dictating the nuclearity/aggregation and acetate coordination modes of lutidine-coordinated zinc(II) acetate complexes.
Inorg. Chem. 49(1) , 62-72, (2010) The reactions of Zn(OAc)(2).2H(2)O with various positional isomers of lutidine were explored with a view to understand the factors responsible for the nuclearity/aggregation and acetate coordination modes of the products. The reactions of Zn(OAc)(2).2H(2)O wi... |
|
|
Synthesis and anti-inflammatory activity of polyazaheterocyclic derivatives of 6-amino-2,4-lutidine and their precursors.
Arzneimittelforschung 47(5) , 635-42, (1997) Derivatives of N-(4,6-pyridin-2-yl)arylcarboxamides resulting from the integration of the amidic function into 4H-1,2,4-triazole, triazol-3(2H)-one and 1H-tetrazole rings were evaluated as potential anti-inflammatory compounds. The level of activity decreased... |