FORMAMIDOXIME
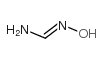
FORMAMIDOXIME structure
|
Common Name | FORMAMIDOXIME | ||
|---|---|---|---|---|
| CAS Number | 624-82-8 | Molecular Weight | 60.05530 | |
| Density | 1.29g/cm3 | Boiling Point | 224ºC at 760 mmHg | |
| Molecular Formula | CH4N2O | Melting Point | 112-115ºC(lit.) | |
| MSDS | Chinese USA | Flash Point | 89.3ºC | |
| Symbol |


GHS07, GHS08 |
Signal Word | Warning | |
|
Antitumor activity of amidoximes (hydroxyurea analogs) in murine tumor systems.
Cancer Res. 38(5) , 1291-5, (1978) A series of amidoximes was prepared and evaluated for possible antitumor activity against L1210 leukemia. Three of the most active compounds in the L1210 system, formamidoxime, acetamidoxime, and 2-aminoacetamidoxime hydrochloride, were also active against P3... |
|
|
Nitric oxide synthesis by tracheal smooth muscle cells by a nitric oxide synthase-independent pathway.
Am. J. Physiol. 275(5 Pt 1) , L895-901, (1998) Nitric oxide (NO) is known to be synthesized from L-arginine in a reaction catalyzed by NO synthase. Liver cytochrome P-450 enzymes also catalyze the oxidative cleavage of C==N bonds of compounds containing a -C(NH2)==NOH function, producing NO in vitro. The ... |
|
|
Effects of phenobarbital, beta-naphthoflavone, dexamethasone, and formamidoxime on the turnover of inducible microsomal proteins in cultured hepatocytes.
J. Biol. Chem. 256(24) , 13079-84, (1981) Microsomal proteins from cultured chick embryo hepatocytes were separated by polyacrylamide gel electrophoresis and their rate constants of degradation (Kd) were estimated using double isotope techniques. The proteins were found to be heterogeneous in their t... |
|
|
Controlled synthesis and alkaline earth ion binding of switchable formamidoxime-based crown ether analogs.
Chem. Commun. (Camb.) 48(63) , 7829-31, (2012) The partial positive charge of amide protons is used to promote macrocyclization and form crown-ether analogs. Their deprotonation generates very selective pH-switchable alkaline earth ion receptors only in the presence of an appropriate substrate. |
|
|
Z-formamidoximes in molecular folding and macrocycles.
Org. Biomol. Chem. 9(22) , 7647-51, (2011) The formamidoxime configurational Z isomer coupled with the pyridylbiscarboxamide conformational codon were used to fold planar, curved structures. When embedded into macrocycles, this folded motif promotes dimerization through π-π stacking and hydrogen-bondi... |