Orlistat
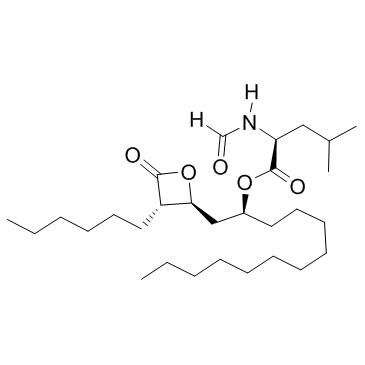
Orlistat structure
|
Common Name | Orlistat | ||
|---|---|---|---|---|
| CAS Number | 96829-58-2 | Molecular Weight | 495.735 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 615.9±30.0 °C at 760 mmHg | |
| Molecular Formula | C29H53NO5 | Melting Point | <50ºC | |
| MSDS | Chinese USA | Flash Point | 326.3±24.6 °C | |
|
Orlistat and calcium oxalate crystalluria: an association that needs consideration.
Ren. Fail. 32(8) , 1019-21, (2010) Obesity is currently an epidemic across the globe. Obese patients unable to achieve significant weight loss with lifestyle changes alone may require drug therapy. Clinical trials have shown that orlistat administration may not only lead to weight loss but als... |
|
|
Orlistat-induced oxalate nephropathy may be dose-independent and present as a late manifestation.
J. La. State Med. Soc. 165(5) , 283-5, (2013) We present the case of a 61-year-old Caucasian male veteran who had been on orlistat (120mg dosing) for four years, and had changed to the over-the-counter (OTC) form, Alli (orlistat 60mg), about three months before presentation. He had been experiencing naus... |
|
|
Mechanisms of cholesterol and saturated fatty acid lowering by Quillaja saponaria extract, studied by in vitro digestion model.
Food Funct. 6 , 1319-30, (2015) Quillaja saponin extracts are known to reduce plasma cholesterol levels in humans. Here we study the mechanism of this effect with Quillaja Dry saponin extract (QD). In vitro model of triglyceride lipolysis is used to quantify the effect of QD on the solubili... |
|
|
Obesity drug therapy.
Minerva Endocrinol. 38(3) , 245-54, (2013) Obesity is a chronic disease, and it requires chronic therapy. Hypertension, dyslipidemia, diabetes and cardiovascular diseases are leading causes of mortality in the modern world. All of them are strongly linked to obesity. While treating obesity, those cond... |
|
|
Orlistat for the management of overweight individuals and obesity: a review of potential for the 60-mg, over-the-counter dosage.
Expert Opin. Pharmacother. 8(11) , 1733-42, (2007) Orlistat, in the 60-mg over-the-counter dose, was recently approved by the FDA. This lipase inhibitor blocks absorption of ~25% of ingested fat and has ~85% of the efficacy of the 120-mg dose for weight loss. Over 16 weeks weight loss with diet and orlistat 6... |
|
|
The use of orlistat in the treatment of obesity, dyslipidaemia and Type 2 diabetes.
Expert Opin. Pharmacother. 6(14) , 2483-91, (2005) Orlistat (tetrahydrolipstatin) is an inhibitor of gastrointestinal lipases, especially pancreatic lipase. It is used as an adjunct to diet and exercise in order to achieve weight loss in obese individuals (body mass index > 30 kg/m2) or in overweight individu... |
|
|
Systematic review of the clinical efficacy of sibutramine and orlistat in weigth loss, quality of life and its adverse effects in obese adolescents.
Nutr. Hosp. 26(3) , 451-7, (2011) The prevalence of obesity, a serious public health problem, is increasing among teenagers and thus also increases cardiovascular morbidity and mortality in adulthood.To provide a systematic review of the best evidence about the effect of sibutramine and orlis... |
|
|
The first decade of sibutramine and orlistat: a reappraisal of their expanding roles in the treatment of obesity and associated conditions.
Arq. Bras. Endocrinol. Metabol. 53(2) , 262-70, (2009) Ancillary therapies for weight management, consisting mainly of diet and exercise programs that incorporate variable levels of lifestyle modification techniques, are frequently ineffective to achieve clinically meaningful weight loss and maintenance. Although... |
|
|
Drug treatments for obesity: orlistat, sibutramine, and rimonabant.
Lancet 369(9555) , 71-7, (2007) Antiobesity treatment is recommended for selected patients in whom lifestyle modification is unsuccessful. Two antiobesity drugs are currently licensed for long-term use. Orlistat, a gastrointestinal lipase inhibitor, reduces weight by around 3 kg on average ... |
|
|
Long-term changes in blood pressure following orlistat and sibutramine treatment: a meta-analysis.
Obes. Rev. 11(11) , 777-91, (2010) Previous meta-analyses investigating blood pressure effects of anti-obesity drugs have included studies using non-licensed doses, but not data from head-to-head studies. Furthermore, although diabetes is an important comorbidity in obesity, variation in blood... |