6-Aminocaproic acid
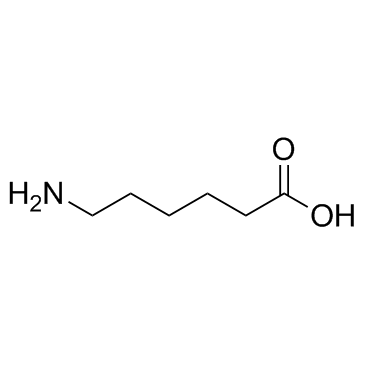
6-Aminocaproic acid structure
|
Common Name | 6-Aminocaproic acid | ||
|---|---|---|---|---|
| CAS Number | 60-32-2 | Molecular Weight | 131.173 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 255.6±23.0 °C at 760 mmHg | |
| Molecular Formula | C6H13NO2 | Melting Point | 207-209 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 108.4±22.6 °C | |
|
Novel Antibodies Reactive with Sialyl Lewis X in Both Humans and Mice Define Its Critical Role in Leukocyte Trafficking and Contact Hypersensitivity Responses.
J. Biol. Chem. 290 , 15313-26, (2015) Sialyl Lewis X (sLe(x)) antigen functions as a common carbohydrate determinant recognized by all three members of the selectin family. However, its expression and function in mice remain undefined due to the poor reactivity of conventional anti-sLe(x) monoclo... |
|
|
Dietary magnesium restriction reduces amygdala-hypothalamic GluN1 receptor complex levels in mice.
Brain Struct. Funct. 220 , 2209-21, (2015) Reduced daily intake of magnesium (Mg(2+)) is suggested to contribute to depression. Indeed, preclinical studies show dietary magnesium restriction (MgR) elicits enhanced depression-like behaviour establishing a causal relationship. Amongst other mechanisms, ... |
|
|
Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species.
Chem. Res. Toxicol. 23 , 171-83, (2010) Drug-induced liver injury is one of the main causes of drug attrition. The ability to predict the liver effects of drug candidates from their chemical structures is critical to help guide experimental drug discovery projects toward safer medicines. In this st... |
|
|
Translating clinical findings into knowledge in drug safety evaluation--drug induced liver injury prediction system (DILIps).
J. Sci. Ind. Res. 65(10) , 808, (2006) Drug-induced liver injury (DILI) is a significant concern in drug development due to the poor concordance between preclinical and clinical findings of liver toxicity. We hypothesized that the DILI types (hepatotoxic side effects) seen in the clinic can be tra... |
|
|
Developing structure-activity relationships for the prediction of hepatotoxicity.
Chem. Res. Toxicol. 23 , 1215-22, (2010) Drug-induced liver injury is a major issue of concern and has led to the withdrawal of a significant number of marketed drugs. An understanding of structure-activity relationships (SARs) of chemicals can make a significant contribution to the identification o... |
|
|
A predictive ligand-based Bayesian model for human drug-induced liver injury.
Drug Metab. Dispos. 38 , 2302-8, (2010) Drug-induced liver injury (DILI) is one of the most important reasons for drug development failure at both preapproval and postapproval stages. There has been increased interest in developing predictive in vivo, in vitro, and in silico models to identify comp... |
|
|
Chemical genetics reveals a complex functional ground state of neural stem cells.
Nat. Chem. Biol. 3(5) , 268-273, (2007) The identification of self-renewing and multipotent neural stem cells (NSCs) in the mammalian brain holds promise for the treatment of neurological diseases and has yielded new insight into brain cancer. However, the complete repertoire of signaling pathways ... |
|
|
Capillary electrophoresis fingerprinting of 8-aminopyrene-1,3,6-trisulfonate derivatized nitrocellulose after partial acid depolymerization.
J. Chromatogr. A. 1387 , 134-43, (2015) Fine characterization of nitrocellulose (NC) remains a challenge, especially in forensic analysis, and a strategy consisting in obtaining representative fingerprints by a separation technique, as for proteins, is of prime interest. In this work, we first esta... |
|
|
Structure elucidation and quantification of impurities formed between 6-aminocaproic acid and the excipients citric acid and sorbitol in an oral solution using high-resolution mass spectrometry and nuclear magnetic resonance spectroscopy.
J. Pharm. Biomed. Anal. 107 , 333-40, (2015) Concentrated solutions containing 6-aminocaproic acid and the excipients citric acid and sorbitol have been studied at temperatures of 50°C, 60°C, 70°C and 80°C as well as at 20°C. It has previously been reported that the commonly employed citric acid is a re... |
|
|
Peptide dimethylation: fragmentation control via distancing the dimethylamino group.
J. Am. Soc. Mass Spectrom. 25(10) , 1694-704, (2014) Direct reductive methylation of peptides is a common method for quantitative proteomics. It is an active derivatization technique; with participation of the dimethylamino group, the derivatized peptides preferentially release intense a1 ions. The advantageous... |