Desloratadine
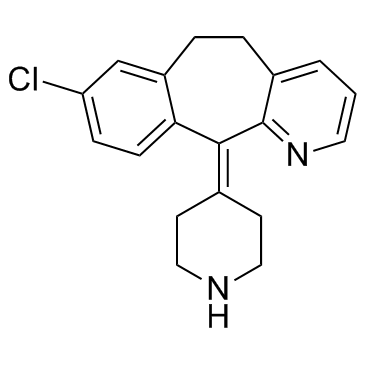
Desloratadine structure
|
Common Name | Desloratadine | ||
|---|---|---|---|---|
| CAS Number | 100643-71-8 | Molecular Weight | 310.821 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 467.9±45.0 °C at 760 mmHg | |
| Molecular Formula | C19H19ClN2 | Melting Point | 150-151°C | |
| MSDS | Chinese USA | Flash Point | 236.8±28.7 °C | |
|
Utility of cerebrospinal fluid drug concentration as a surrogate for unbound brain concentration in nonhuman primates.
Drug Metab. Pharmacokinet. 29(5) , 419-26, (2014) In central nervous system drug discovery, cerebrospinal fluid (CSF) drug concentration (C(CSF)) has been widely used as a surrogate for unbound brain concentrations (C(u,brain)). However, previous rodent studies demonstrated that when drugs undergo active eff... |
|
|
A long-standing mystery solved: the formation of 3-hydroxydesloratadine is catalyzed by CYP2C8 but prior glucuronidation of desloratadine by UDP-glucuronosyltransferase 2B10 is an obligatory requirement.
Drug Metab. Dispos. 43(4) , 523-33, (2015) Desloratadine (Clarinex), the major active metabolite of loratadine (Claritin), is a nonsedating long-lasting antihistamine that is widely used for the treatment of allergic rhinitis and chronic idiopathic urticaria. For over 20 years, it has remained a myste... |
|
|
Differential thermodynamic driving force of first- and second-generation antihistamines to determine their binding affinity for human H1 receptors.
Biochem. Pharmacol. 91(2) , 231-41, (2014) Differential binding sites for first- and second-generation antihistamines were indicated on the basis of the crystal structure of human histamine H1 receptors. In this study, we evaluated differences between the thermodynamic driving forces of first- and sec... |
|
|
Development and validation of an ultra-performance liquid chromatography method for simultaneous analysis of 20 antihistaminics in dietary supplements.
Biomed. Chromatogr. 29(3) , 465-74, (2015) The purpose of this study was to develop and validate an ultra-performance liquid chromatography method for simultaneous analysis of 20 antihistamines (illegal additives) in dietary supplements. The limits of detection and quantitation of the method ranged fr... |
|
|
Prevalence of Desloratadine Slow-metabolizer Phenotype and Food-dependent Pharmacokinetics of Desloratadine in Healthy Chinese Volunteers.
Clin. Drug Investig. 35 , 807-13, (2015) Desloratadine, the major active metabolite of loratadine, is a non-sedating long-acting antihistamine that is widely used in the treatment of allergic rhinitis and chronic idiopathic urticaria. This study aimed to investigate the prevalence of desloratadine s... |
|
|
A novel model of IgE-mediated passive pulmonary anaphylaxis in rats.
PLoS ONE 9(12) , e116166, (2014) Mast cells are central effector cells in allergic asthma and are augmented in the airways of asthma patients. Attenuating mast cell degranulation and with it the early asthmatic response is an important intervention point to inhibit bronchoconstriction, plasm... |
|
|
Astroglial glutamate transporter deficiency increases synaptic excitability and leads to pathological repetitive behaviors in mice.
Neuropsychopharmacology 40 , 1569-79, (2015) An increase in the ratio of cellular excitation to inhibition (E/I ratio) has been proposed to underlie the pathogenesis of neuropsychiatric disorders, such as autism spectrum disorders (ASD), obsessive-compulsive disorder (OCD), and Tourette's syndrome (TS).... |
|
|
Further Characterization of the Metabolism of Desloratadine and Its Cytochrome P450 and UDP-glucuronosyltransferase Inhibition Potential: Identification of Desloratadine as a Relatively Selective UGT2B10 Inhibitor.
Drug Metab. Dispos. 43 , 1294-302, (2015) Desloratadine (Clarinex), the major active metabolite of loratadine (Claritin), is a nonsedating antihistamine used for the treatment of seasonal allergies and hives. Previously we reported that the formation of 3-hydroxydesloratadine, the major human metabol... |