Diosgenin
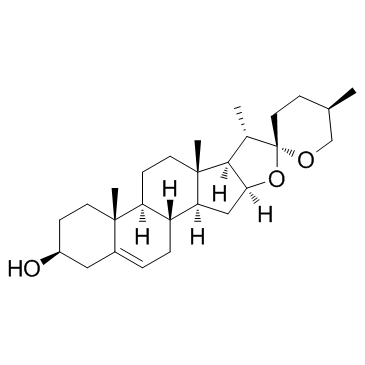
Diosgenin structure
|
Common Name | Diosgenin | ||
|---|---|---|---|---|
| CAS Number | 512-04-9 | Molecular Weight | 414.621 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 527.1±50.0 °C at 760 mmHg | |
| Molecular Formula | C27H42O3 | Melting Point | 205-208°C | |
| MSDS | Chinese USA | Flash Point | 272.6±30.1 °C | |
|
Diosgenin-based thio(seleno)ureas and triazolyl glycoconjugates as hybrid drugs. Antioxidant and antiproliferative profile.
Eur. J. Med. Chem. 99 , 67-81, (2015) The stereoselective preparation of diosgenin-derived thio(seleno)ureas and glycomimetics bearing a 1,2,3-triazolyl tether on C-3 has been accomplished. The key steps in the synthetic pathway are the incorporation of an amino moiety and its further transformat... |
|
|
In Vivo Protective Effects of Diosgenin against Doxorubicin-Induced Cardiotoxicity.
Nutrients 7 , 4938-54, (2015) Doxorubicin (DOX) induces oxidative stress leading to cardiotoxicity. Diosgenin, a steroidal saponin of Dioscorea opposita, has been reported to have antioxidant activity. Our study was aimed to find out the protective effect of diosgenin against DOX-induced ... |
|
|
The Chinese herb polyphyllin D sensitizes ovarian cancer cells to cisplatin-induced growth arrest.
J. Cancer Res. Clin. Oncol. 141(2) , 237-42, (2015) We evaluated the effects of polyphyllin D (PD), a natural compound with anti-neoplastic activity and a major component of the Chinese herb Paris polyphylla, on ovarian cancer (OVCA) cell line proliferation and platinum sensitivity.A panel of 20 OVCA cell line... |
|
|
Diacetoxyiodobenzene-mediated synthesis of unnatural furospirostane sapogenins derived from diosgenin and tigogenin.
Steroids 78(9) , 798-802, (2013) Two unnatural steroid sapogenins bearing a furospirostane side chain were prepared starting from the readily available spirostane sapogenins, tigogenin and diosgenin following a synthetic protocol that included: (i) introduction of a carbonyl group at positio... |
|
|
Dioscin inhibits colon tumor growth and tumor angiogenesis through regulating VEGFR2 and AKT/MAPK signaling pathways.
Toxicol. Appl. Pharmacol. 281(2) , 166-73, (2014) Dioscin has shown cytotoxicity against cancer cells, but its in vivo effects and the mechanisms have not elucidated yet. The purpose of the current study was to assess the antitumor effects and the molecular mechanisms of dioscin. We showed that dioscin could... |
|
|
Diosgenin, a steroidal saponin, exhibits anticancer activity by attenuating lipid peroxidation via enhancing antioxidant defense system during NMU-induced breast carcinoma.
J. Environ. Pathol. Toxicol. Oncol. 31(2) , 121-9, (2012) Diosgenin, a natural steroidal saponin, has been reported to be found predominantly in fenugreek and has diverse biological properties. N-Methyl-N-nitrosourea (NMU) is a mammary gland-specific carcinogen that closely mimics human breast cancer in many aspects... |
|
|
Diosgenin inhibits the migration of human breast cancer MDA-MB-231 cells by suppressing Vav2 activity
Phytomedicine 21(6) , 871-6, (2014) Diosgenin, a naturally occurring steroidal saponin, possess tumor therapeutic potential. However, the effect of diosgenin on cancer metastasis remains poorly understood. In this study, we performed in vitro experiments to investigate the inhibitory activity o... |
|
|
Effects of Jamaican bitter yam (Dioscorea polygonoides) and diosgenin on blood and fecal cholesterol in rats.
J. Med. Food 17(11) , 1183-8, (2014) A sapogenin-rich preparation from Jamaican bitter yam (Dioscorea polygonoides) has been shown to reduce blood cholesterol concentrations in hypercholesterolemic rats and mice. Also, diosgenin supplementation has been reported to have antilipemic effects in se... |
|
|
Inhibitory effects of chickpea and Tribulus terrestris on lipase, α-amylase and α-glucosidase.
Food Chem. 205 , 163-9, (2016) The total saponin content and its in vitro bioaccessibilities in Tribulus terrestris and chickpea were determined by a static in vitro digestion method (COST FA1005 Action INFOGEST). Also, in vitro inhibitory effects of the chosen food samples on lipid and st... |
|
|
Synthesis of novel anticancer agents through opening of spiroacetal ring of diosgenin.
Steroids 87 , 108-18, (2014) Diosgenin has been modified to furostane derivatives after opening the F-spiroacetal ring. The aldehyde group at C26 in derivative 8 was unexpectedly transformed to the ketone 9. The structure of ketone 9 was confirmed by spectroscopy and finally by X-ray cry... |