| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
α-Amylase
CAS:9000-90-2 |
|
 |
Pepsin
CAS:9001-75-6 |
|
 |
Pancreatin
CAS:8049-47-6 |
|
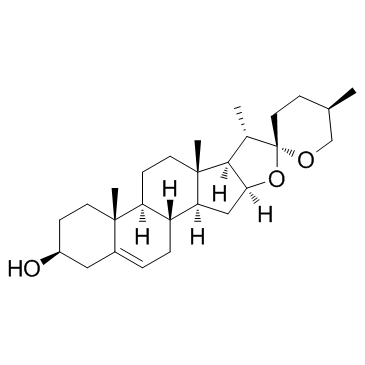 |
Diosgenin
CAS:512-04-9 |
|
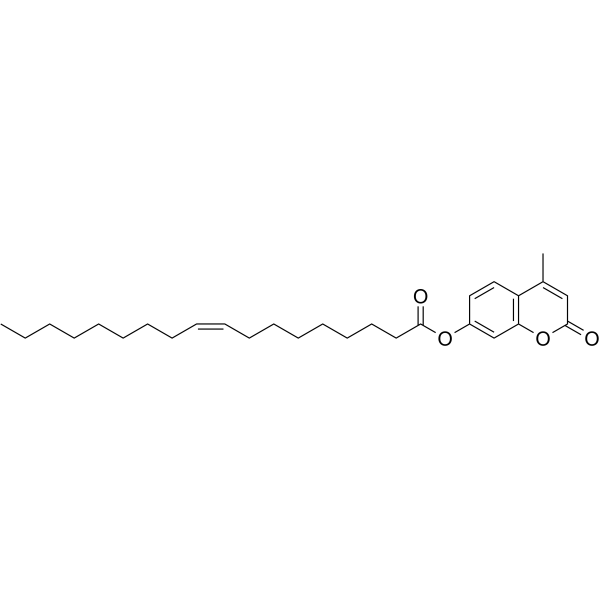 |
4-Methylumbelliferyl Oleate
CAS:18323-58-5 |