Span 80
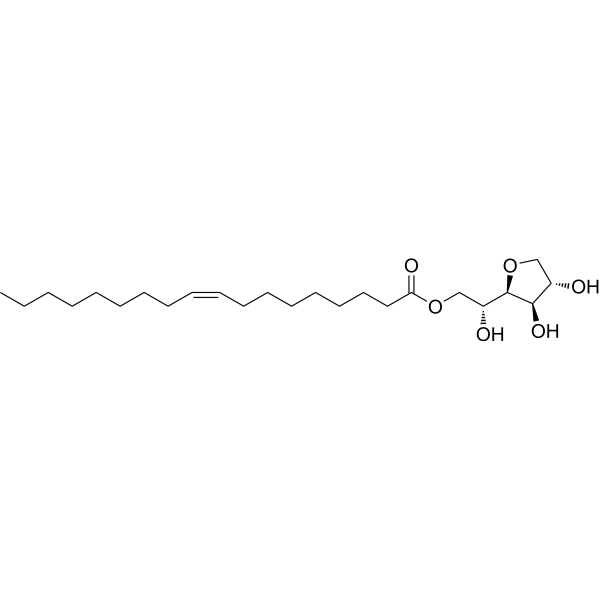
Span 80 structure
|
Common Name | Span 80 | ||
|---|---|---|---|---|
| CAS Number | 1338-43-8 | Molecular Weight | 428.603 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 579.3±50.0 °C at 760 mmHg | |
| Molecular Formula | C24H44O6 | Melting Point | 0.986ºC | |
| MSDS | Chinese USA | Flash Point | 186.2±23.6 °C | |
|
Cytokine pathway disruption in a mouse model of schizophrenia induced by Munc18-1a overexpression in the brain.
J. Neuroinflammation 11 , 128, (2014) An accumulating body of evidence points to the significance of neuroinflammation and immunogenetics in schizophrenia, and an imbalance of cytokines in the central nervous system (CNS) has been suggested to be associated with the disorder. Munc18-overexpressin... |
|
|
Maltodextrin fast dissolving films for quercetin nanocrystal delivery. A feasibility study.
Carbohydr. Polym. 121 , 217-23, (2015) The objective of this study was to evaluate the feasibility to prepare fast dissolving films as quercetin nanocrystal delivery systems, using maltodextrins as film forming material and glycerin as plasticizer, with the goal of enhancing quercetin oral bioavai... |
|
|
Anionic nanoparticles based on Span 80 as low-cost, simple and efficient non-viral gene-transfection systems.
Int. J. Pharm. 476(1-2) , 23-30, (2015) The existing strategies in the design of non-viral vectors for gene therapy are primarily conceived for cationic systems. However, the safety concerns associated with the use of positively charged systems for nucleic acid delivery and several reports regardin... |
|
|
Biochemistry of lipolytic enzymes secreted by Penicillium solitum and Cladosporium cladosporioides.
Biosci. Biotechnol. Biochem. 78(2) , 245-54, (2014) Two distinct extracellular lipases were obtained from Penicillium solitum 194A, isolated from domestic compost, and Cladosporium cladosporioides 194B, isolated from dairy wastewater. These alkaline enzymes had molecular masses of 42 and 30 kDa, respectively. ... |
|
|
In vitro release testing methods for vitamin E nanoemulsions.
Int. J. Pharm. 475(1-2) , 393-400, (2014) This study reports the release properties of the poorly water-soluble active vitamin E acetate from oil/water nanoemulsions containing canola oil, CremophorRH40(®) and Span80(®) prepared using a low energy emulsification method (EPI process). Drug release was... |
|
|
Nanoemulsions (NEs), liposomes (LPs) and solid lipid nanoparticles (SLNs) for retinyl palmitate: effect on skin permeation.
Int. J. Pharm. 473(1-2) , 591-8, (2014) The aim of this study was to develop biocompatible lipid-based nanocarriers for retinyl palmitate (RP) to improve its skin delivery, photostability and biocompatibility, and to avoid undesirable topical side effects. RP loaded nanoemulsions (NEs), liposomes (... |
|
|
Lecithin-linker formulations for self-emulsifying delivery of nutraceuticals.
Int. J. Pharm. 471(1-2) , 92-102, (2014) Lecithin-linker microemulsions are formulations produced with soybean lecithin in combination with a highly lipophilic (lipophilic linker) and highly hydrophilic (hydrophilic linkers) surfactant-like additives. In this work, lecithin-linker systems were formu... |
|
|
Evidence for the existence of powder sub-populations in micronized materials: aerodynamic size-fractions of aerosolized powders possess distinct physicochemical properties.
Pharm. Res. 31(12) , 3251-64, (2014) To investigate the agglomeration behaviour of the fine (<5.0 μm) and coarse (>12.8 μm) particle fractions of salmeterol xinafoate (SX) and fluticasone propionate (FP) by isolating aerodynamic size fractions and characterising their physicochemical and re-disp... |
|
|
Development of tumor-specific caffeine-potentiated chemotherapy using a novel drug delivery system with Span 80 nano-vesicles.
Oncol. Rep. 33(4) , 1593-8, (2015) In recent years, chemotherapy with caffeine has manifested potently high efficacy against osteosarcoma, although adverse effects have been observed. Recently, we developed a novel drug delivery system (DDS) with nonionic vesicles prepared from Span 80 which h... |
|
|
Lipid based nanocarrier system for the potential oral delivery of decitabine: formulation design, characterization, ex vivo, and in vivo assessment.
Int. J. Pharm. 477(1-2) , 601-12, (2014) The aim of this study was to design and fabricate nanostructured lipid carrier (NLC) for the potential oral delivery of decitabine (DCB). NLC was prepared by cold homogenization technique and optimized by the Box-Behnken experimental design. It was further ch... |