1-phenyl-N-propylpropan-2-amine
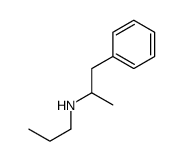
1-phenyl-N-propylpropan-2-amine structure
|
Common Name | 1-phenyl-N-propylpropan-2-amine | ||
|---|---|---|---|---|
| CAS Number | 51799-32-7 | Molecular Weight | 177.28600 | |
| Density | 0.897g/cm3 | Boiling Point | 254.4ºC at 760mmHg | |
| Molecular Formula | C12H19N | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 103.6ºC | |
| Symbol |



GHS02, GHS06, GHS08 |
Signal Word | Danger | |
|
A convenient derivatization method for gas chromatography/mass spectrometric determination of phenmetrazine in urine using 2,2,2-trichloroethyl chloroformate.
J. Forensic Sci. 43(3) , 630-5, (1998) Phenmetrazine is a central nervous system stimulant currently used as an anorectic agent. The drug is abused and is reported to cause death from overdose. We describe a new derivatization method for phenmetrazine using 2,2,2-trichloroethyl chloroformate. Quan... |
|
|
Amphetamine in rat brain after intraperitoneal injection of N-alkylated analogues.
Prog. Neuropsychopharmacol. Biol. Psychiatry 7(4-6) , 813-6, (1983) Three N-alkylated analogues of amphetamine were administered intraperitoneally to male Sprague-Dawley rats and whole brain levels of amphetamine (AM) and the N-alkyl analogue were determined one hour after injection of the N-alkylated compounds. The drugs adm... |
|
|
In vivo and in vitro o-methylation of 1-(3,4-dihydroxyphenyl)-2-(n-propyl-amino) propane - an intermediate in N-(N-propyl) amphetamine metabolism.
Res. Commun. Chem. Pathol. Pharmacol. 36(1) , 173-6, (1982) In vitro metabolism of 1-(3,4-dihydroxyphenyl)-2-(n-propylamino)-propane (Id) by rat liver cytosol in the presence of S-adenosyl-L-methyl-methionine produced almost quantitatively the corresponding 3-methoxy metabolite. 1-(4-Hydroxy-3-methoxyphenyl)-2-(n-prop... |
|
|
Structure-activity relationships among some d-N-alkylated amphetamines.
Pharmacol. Biochem. Behav. 13(6) , 869-76, (1980) d-N-Alkylated amphetamines were synthesized in a series up to and including d-N-butylamphetamine and potencies of these compounds were compared in (1) rhesus monkeys allowed to respond for intravenous infusions of the drugs, (2) rats allowed to drink a milk s... |