Ethynyl 4-methylphenyl sulfone
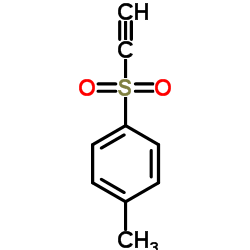
Ethynyl 4-methylphenyl sulfone structure
|
Common Name | Ethynyl 4-methylphenyl sulfone | ||
|---|---|---|---|---|
| CAS Number | 13894-21-8 | Molecular Weight | 180.224 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 297.9±33.0 °C at 760 mmHg | |
| Molecular Formula | C9H8O2S | Melting Point | 73-74ºC(lit.) | |
| MSDS | Chinese USA | Flash Point | 155.6±18.1 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Tosvinyl and besvinyl as protecting groups of imides, azinones, nucleosides, sultams, and lactams. Catalytic conjugate additions to tosylacetylene.
J. Org. Chem. 79(18) , 8826-34, (2014) The use of the 2-(4-methylphenylsulfonyl)ethenyl (tosvinyl, Tsv) group for the protection of the NH group of a series of imides, azinones (including AZT), inosines, and cyclic sulfonamides has been examined. The Tsv-protected derivatives are obtained in excel... |
|
|
Efficient and practical method for synthesizing optically active indan-2-ols by the Ti(O-i-Pr)(4)/2 i-PrMgCl-mediated metalative Reppe reaction.
J. Org. Chem. 68(12) , 4980-3, (2003) An efficient and practical synthesis of optically active indan-2-ols 1 has been developed starting from readily accessible optically active 4-siloxy-1,6-alkadiynes 2 and ethynyl p-tolyl sulfone, where the metalative Reppe reaction mediated by an economical di... |
|
|
A Simplified Method for the Preparation of Ethynyl P-Tolyl Sulfone and Ethynyl Phenyl Sulfone. Chen Z and Trudell ML.
Synth. Commun. 24(21) , 3149-55, (1994)
|
|
|
Design and synthesis of beta-methoxyacrylate analogues via click chemistry and biological evaluations.
Bioorg. Med. Chem. Lett. 17 , 1979-83, (2007) A library of potential antifungal triazole-modified beta-methoxyacrylate analogues was designed and synthesized via a Cu(I)-catalyzed 1,3-dipolar alkyne-azide coupling reaction or 'click chemistry'. Subsequent biological screening revealed that some compounds... |