Digalacturonic acid
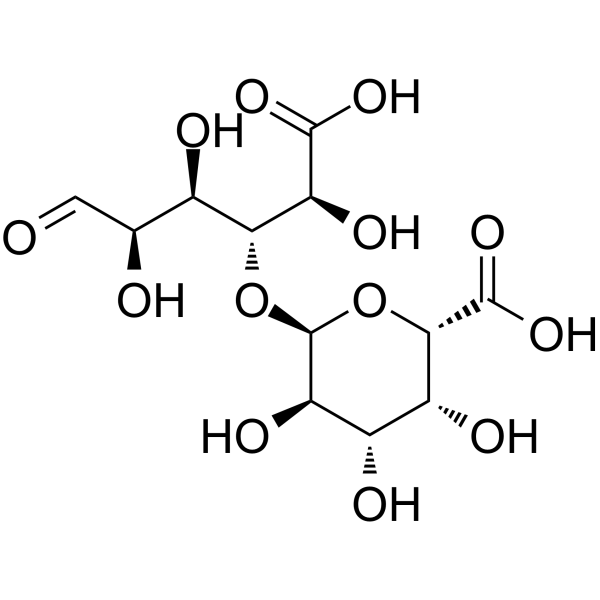
Digalacturonic acid structure
|
Common Name | Digalacturonic acid | ||
|---|---|---|---|---|
| CAS Number | 5894-59-7 | Molecular Weight | 370.26400 | |
| Density | 1.97 g/cm3 | Boiling Point | 793.8ºC at 760 mmHg | |
| Molecular Formula | C12H18O13 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 296.6ºC | |
|
Molecular characterization of a thermophilic endo-polygalacturonase from Thielavia arenaria XZ7 with high catalytic efficiency and application potential in the food and feed industries.
J. Agric. Food Chem. 62(52) , 12686-94, (2015) Thermophilic endo-polygalacturonases with high catalytic efficiency are of great interest in the food and feed industries. This study identified an endo-polygalacturonase gene (pg7fn) of glycoside hydrolase family 28 in the thermophilic fungus Thielavia arena... |
|
|
Horizontal Gene Transfer of Pectinases from Bacteria Preceded the Diversification of Stick and Leaf Insects.
Sci. Rep. 6 , 26388, (2016) Genes acquired by horizontal transfer are increasingly being found in animal genomes. Understanding their origin and evolution requires knowledge about the phylogenetic relationships from both source and recipient organisms. We used RNASeq data and respective... |
|
|
Coordination ability of digalactosamine, and di- and trigalacturonic acids. Potentiometric and spectroscopic studies of Cu(II) complexes.
J. Inorg. Biochem. 57(1) , 1-10, (1995) Potentiometric and spectroscopic (EPR, CD, and absorption spectra) data obtained for digalactosamine and di- and trigalacturonic acid with Cu(II) have shown that the di-sugar binding is usually less efficient than that of monomeric units while the tri-sugar c... |
|
|
The D-galacturonic acid catabolic pathway in Botrytis cinerea.
Fungal Genet. Biol. 48(10) , 990-7, (2011) D-galacturonic acid is the most abundant component of pectin, one of the major polysaccharide constituents of plant cell walls. Galacturonic acid potentially is an important carbon source for microorganisms living on (decaying) plant material. A catabolic pat... |
|
|
Specific recognition of saturated and 4,5-unsaturated hexuronate sugars by a periplasmic binding protein involved in pectin catabolism.
J. Mol. Biol. 369 , 759-770, (2007) The process of pectin depolymerization by pectate lyases and glycoside hydrolases produced by pectinolytic organisms, particularly the phytopathogens from the genus Erwinia, is reasonably well understood. Indeed each extracellular and intracellular catabolic ... |
|
|
The structural basis for exopolygalacturonase activity in a family 28 glycoside hydrolase.
J. Mol. Biol. 368 , 1215-1222, (2007) Family 28 glycoside hydrolases (polygalacturonases) are found in organisms across the plant, fungal and bacterial kingdoms, where they are central to diverse biological functions such as fruit ripening, biomass recycling and plant pathogenesis. The structures... |
|
|
Identification of TogMNAB, an ABC transporter which mediates the uptake of pectic oligomers in Erwinia chrysanthemi 3937.
Mol. Microbiol. 41 , 1113-1123, (2001) The bacterium Erwinia chrysanthemi, which causes soft rot disease on various plants, is able to use pectin as a carbon source for growth. Knowledge of the critical step in pectin catabolism which allows the entry of pectic oligomers into the cells is scarce. ... |
|
|
Use of the Saccharomyces cerevisiae endopolygalacturonase promoter to direct expression in Escherichia coli.
J. Ind. Microbiol. Biotechnol. 39(7) , 1023-9, (2012) In Saccharomyces cerevisiae, an endopolygalacturonase encoded by the PGL1 gene catalyzes the random hydrolysis of the α-1,4 glycosidic linkages in polygalacturonic acid. To study the regulation of the PGL1 gene, we constructed a reporter vector containing the... |
|
|
High-resolution structure of proteinase K cocrystallized with digalacturonic acid.
Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 65 , 192-198, (2009) Proteinase K, a subtilisin-like fungal protease, was crystallized from a cocktail of small molecules containing digalacturonic acid (DGA). The crystal structure was determined to 1.32 A resolution and refined to an R factor of 0.158. The final model contained... |
|
|
Production, characterization, and identification using proteomic tools of a polygalacturonase from Fusarium graminearum.
J. Basic Microbiol. , doi:10.1002/jobm.201300651, (2014) Since enzymatic degradation is a mechanism or component of the aggressiveness of a pathogen, enzymatic activities from a Fusarium graminearum isolate obtained from infected wheat spikes of Argentina Pampa region were studied in order to understand the disease... |