Calcium cyanamide
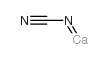
Calcium cyanamide structure
|
Common Name | Calcium cyanamide | ||
|---|---|---|---|---|
| CAS Number | 156-62-7 | Molecular Weight | 80.10210 | |
| Density | 2.29 | Boiling Point | 258.5ºC at 760 mmHg | |
| Molecular Formula | CCaN2 | Melting Point | >300 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 42.1ºC | |
| Symbol |



GHS02, GHS05, GHS07 |
Signal Word | Danger | |
|
Synthesis of novel phosphorylated guanidine derivatives from cyanamide and their anti-inflammatory activity.
Chem. Pharm. Bull. 61(1) , 25-32, (2013) A series of novel guanidine derivatives were synthesized in three steps and their anti-inflammatory activities in vitro and in vivo evaluated. 2-Aminopyridin-3-ol (1) was reacted with thiophosphoryl chloride (2) to give a monochloride (3). It was further reac... |
|
|
Quantification of canavanine, 2-aminoethanol, and cyanamide in Aphis craccivora and its host plants, Robinia pseudoacacia and Vicia angustifolia: effects of these compounds on larval survivorship of Harmonia axyridis.
J. Chem. Ecol. 38(12) , 1552-60, (2012) The cowpea aphid Aphis craccivora that infests the black locust Robinia pseudoacacia shows toxicity to its predator, the multicolored Asian ladybird beetle, Harmonia axyridis. In contrast, the same aphid species that infests the common vetch, Vicia angustifol... |
|
|
Opposite motor responses elicited by ethanol in the posterior VTA: the role of acetaldehyde and the non-metabolized fraction of ethanol.
Neuropharmacology 72 , 204-14, (2013) Recent electrophysiological evidence suggests that ethanol simultaneously exerts opposite effects on the activity of dopamine (DA) neurons in the ventral tegmental area (VTA) through two parallel mechanisms, one promoting and the other reducing the GABA relea... |
|
|
[Cyanamide-ethanol reaction induced shock: report of a case and literature review].
Chudoku. Kenkyu. 26(4) , 295-9, (2013) Cyanamide is a known alcohol deterrent, and it may cause severe cyanamide-ethanol reaction if a patient consumes high amounts of alcohol during treatment. We report a rare case of cyanamide-ethanol reaction-induced shock in a 73-year-old man who was taking cy... |
|
|
Cyanamide-mediated Inhibition of N-acetyltransferase 1.
Toxicology 302(1) , 1-10, (2012) Cyanamide has been used for decades for medical intentions in the treatment of alcoholism and for agricultural purposes as a plant growth regulator and bud-breaking agent. Its therapeutic effect is mediated by reversible inhibition of aldehyde dehydrogenase a... |
|
|
Iron-catalyzed formation of 2-aminopyridines from diynes and cyanamides.
J. Org. Chem. 77(17) , 7555-63, (2012) Diynes and cyanamides undergo an iron-catalyzed [2 + 2 + 2] cycloaddition to form highly substituted 2-aminopyridines in an atom-efficient manner that is both high yielding and regioselective. This system was also used to cyclize two terminal alkynes and a cy... |
|
|
Analysis of responses to glyceryl trinitrate and sodium nitrite in the intact chest rat
Nitric Oxide 26(4) , 223-8, (2012) Highlights ► Nitroglycerin/glyceryl trinitrate (GTN) has been used for over 140years. ► Mechanism of action remains unknown. ► GTN metabolizes to 1,2-GDN and nitrite anion. ► Nitrite anion derived from GTN does not produce nitric oxide. |
|
|
Inhibition of tomato (Solanum lycopersicum L.) root growth by cyanamide is due to altered cell division, phytohormone balance and expansin gene expression.
Planta 236(5) , 1629-38, (2012) Cyanamide (CA) has been reported as a natural compound produced by hairy vetch (Vicia villosa Roth.) and it was shown also to be an allelochemical, responsible for strong allelopathic potential in this species. CA phytotoxicity has been demonstrated on variou... |
|
|
Cyanamide mode of action during inhibition of onion (Allium cepa L.) root growth involves disturbances in cell division and cytoskeleton formation.
Planta 234(3) , 609-21, (2011) Cyanamide is an allelochemical produced by hairy vetch (Vicia villosa Roth.). Its phyotoxic effect on plant growth was examined on roots of onion (Allium cepa L.) bulbs. Water solution of cyanamide (2-10 mM) restricted growth of onion roots in a dose-dependen... |
|
|
Chlorotrimethylsilane activation of acylcyanamides for the synthesis of mono-N-acylguanidines.
J. Org. Chem. 76(16) , 6967-71, (2011) A simple and efficient one-pot method for the synthesis of monoprotected guanidines is presented. Treatment of an acylcyanamide with chlorotrimethylsilane generates a reactive N-silylcarbodiimide capable of guanylating a variety of amines. Typically the react... |