Mefexamide
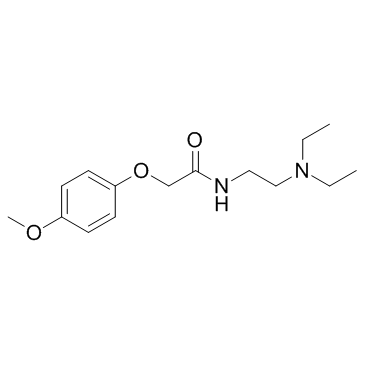
Mefexamide structure
|
Common Name | Mefexamide | ||
|---|---|---|---|---|
| CAS Number | 1227-61-8 | Molecular Weight | 280.36 | |
| Density | 1.0586 (rough estimate) | Boiling Point | 460.4ºC at 760 mmHg | |
| Molecular Formula | C15H24N2O3 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 232.2ºC | |
| Symbol |

GHS06 |
Signal Word | Danger | |
|
[Correction of psychodepressive effects of benzodiazepine tranquilizers by administration of psychostimulants].
Biull. Eksp. Biol. Med. 94(10) , 48-51, (1982)
|
|
|
[Preliminary pharmacological investigations of six new p-chlorphenoxyacetic acid derivatives (author's transl)].
Ann. Univ. Mariae Curie. Sklodowska. Med. 34 , 259-68, (1979)
|
|
|
[Contribution to biotransformation and analysis of the psychopharmacon mefexamide (author's transl)].
Z. Rechtsmed. 87(1-2) , 117-27, (1981) After oral administration of a therapeutic dosage (400 mg) Mefexamide (I), six excretion products were detected in human urine and subsequently identified by GC/MS and TLC: in addition to the unchanged drug (I) in the acidic extracted urine without hydrolysis... |
|
|
[Quantitative determination of mefexamide and its main degradation product desmethyl-mefexamide in human urine after ingestion of therapeutic doses (author's transl)].
Z. Rechtsmed. 87(4) , 297-303, (1981) After oral ingestion of 400 mg Mefexamidehydrochloride for Mefexamide (I) and its main degradation product Desmethyl-Mefexamide (II) the following pharmakokinetic parameters have been determined: 1. Elimination of I and II follows 1st order kinetics. 2. Biolo... |