1,2-aceanthrylenedione
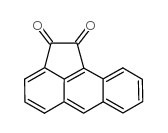
1,2-aceanthrylenedione structure
|
Common Name | 1,2-aceanthrylenedione | ||
|---|---|---|---|---|
| CAS Number | 6373-11-1 | Molecular Weight | 232.23400 | |
| Density | 1.433g/cm3 | Boiling Point | 462.6ºC at 760 mmHg | |
| Molecular Formula | C16H8O2 | Melting Point | 270-273ºC(lit.) | |
| MSDS | Chinese USA | Flash Point | 199.7ºC | |
|
Reactions of Acenaphthenequinone and Aceanthrenequinone with Arenes in Superacid.
Appl. Catal. A Gen. 336(1-2) , 128-132, (2008) The hydroxyalkylation reactions of aceanthrenequinone (6) and acenapthenequinone (7) with a series of arenes have been studied. In reactions with the Br__nsted superacid CF(3)SO(3)H (triflic acid), the condensation products are formed in good yields (58-99%, ... |
|
|
Planarity and constraint of the carbonyl groups in 1,2-diones are determinants for selective inhibition of human carboxylesterase 1.
J. Med. Chem. 50 , 5727-34, (2007) Carboxylesterases (CE) are ubiquitous enzymes responsible for the detoxification of xenobiotics, including numerous clinically used drugs. Therefore, the selective inhibition of these proteins may prove useful in modulating drug half-life and bioavailability.... |
|
|
Deoxygenation of some α-dicarbonyl compounds by tris(diethylamino)phosphine in the presence of fullerene C60.
J. Org. Chem. 76(8) , 2548-57, (2011) The reactions of such cyclic α-diketones as acenaphthenequinone, aceanthrenequinone, and N-alkylisatins, with hexaethyltriaminophosphine in the presence of the fullerene C(60), lead to the formation of methanofullerene derivatives under mild conditions. This ... |
|
|
MacDonald [2 + 2]-type condensation with vicinal diketones: synthesis and properties of novel spiro-tricyclic porphodimethenes.
Org. Lett. 3(15) , 2281-4, (2001) [structure: see text] Acid-catalyzed [2 + 2] condensation reactions of polycyclic aromatic vicinal diketones including aceanthrenequinone, phenathrenequinone, and pyrene-4,5-dione with 5-mesityldipyrromethanes are outlined, and this methodology provides a fle... |