4-Dimethylaminobenzonitrile
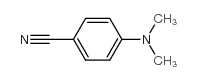
4-Dimethylaminobenzonitrile structure
|
Common Name | 4-Dimethylaminobenzonitrile | ||
|---|---|---|---|---|
| CAS Number | 1197-19-9 | Molecular Weight | 146.18900 | |
| Density | 1.04g/cm3 | Boiling Point | 318 °C(lit.) | |
| Molecular Formula | C9H10N2 | Melting Point | 72-75 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 317-319°C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Predicting the carcinogenicity of the aromatic amine derivatives tested in the second UKEMS Collaborative Study.
Mutagenesis 1(2) , 119-23, (1986) The carcinogenicity prediction and battery selection (CPBS) procedure was used to analyze the short-term in vitro and in vivo genotoxicity results obtained during the Second UKEMS Collaborative Study. In accordance with preliminary animal bioassay results, CP... |
|
|
The formation of a novel mercapturic acid during the metabolism of an N-methyl aromatic amine, 4-cyano-N,N-dimethylaniline.
Biochem. Pharmacol. 33(14) , 2345-6, (1984)
|
|
|
Computer-automated prediction of the mutagenicity of benzidine, 4,4"-diaminoterphenyl, 4-dimethylaminoazobenzene and 4-cyanodimethylaniline: comparison with the results of the Second UKEMS Collaborative Study.
Mutagenesis 1(4) , 275-82, (1986) There was agreement between the experimental results, obtained in the course of the Second UKEMS Collaborative Study, for the mutagenicity in Salmonella typhimurium of benzidine, 4,4"-diaminoterphenyl, 4-dimethylaminoazobenzene and 4-cyanodimethylaniline and ... |
|
|
The fate of 4-cyano-N,N-dimethylaniline in rats; a novel involvement of glutathione in the metabolism of anilines.
Xenobiotica 14(12) , 925-34, (1984) 4-Cyano-N,N-dimethylaniline (CDA), when administered to rats as a single oral dose (18.5 mg/kg), was rapidly absorbed and eliminated as a mixture of metabolites in the urine (86% dose after 24 h). Residues in tissues after 48 h, expressed as microgram equiv. ... |
|
|
Presence and absence of excited state intramolecular charge transfer with the six isomers of dicyano-N,N-dimethylaniline and dicyano-(N-methyl-N-isopropyl)aniline.
J. Phys. Chem. A 115(40) , 10823-45, (2011) The excited state behavior of the six m,n-dicyano-N,N-dimethylanilines (mnDCDMA) and m,n-dicyano-(N-methyl-N-isopropyl)anilines (mnDCMIA) is discussed as a function of solvent polarity and temperature. The dicyano moiety in these electron donor (D)/acceptor (... |
|
|
NMR study of whole rat bile: the biliary excretion of 4-cyano-N,N-dimethyl aniline by an isolated perfused rat liver and a liver in situ.
J. Pharm. Biomed. Anal. 13(6) , 735-45, (1995) The structure of two biliary metabolites of 4-cyano-N,N-dimethyl aniline (CDA) contained in whole rat bile have been studied in detail by NMR at 400 MHz. A 4-cyano-N-methyl glutathione-N-aniline conjugate was identified as a biliary metabolite of CDA using re... |
|
|
The fate of 4-cyano-N,N-dimethylaniline in mice: the occurrence of a novel metabolite during N-demethylation of an aromatic amine.
Xenobiotica 15(5) , 391-7, (1985) 4-Cyano-N,N-dimethylaniline (CDA), when administered as a single oral dose to mice (18.5 mg/kg), was rapidly absorbed and eliminated. The major route of elimination was the urine (78% dose in 24h). The residues in the tissues 48 h after dosing, as microgram e... |
|
|
The fate of 4-cyanoacetanilide in rats and mice; mechanism of formation of a novel electrophilic metabolite.
Xenobiotica 18(8) , 955-66, (1988) 1. The metabolic fate of 4-cyanoacetanilide (CAA), labelled with 14C and 13C in the N-acetyl group, was studied in rats (oral dose, 22.5 mg/kg) and mice (oral dose 21.7 mg/kg). 2. The metabolic profile in the urine of rats was compared with that obtained prev... |
|
|
Comparative chemical mutagenesis; well designed and well assessed. Comparative genetic toxicology: the second UK EMS Collaborative Study.
Mutat. Res. 157(2-3) , 107-10, (1985)
|