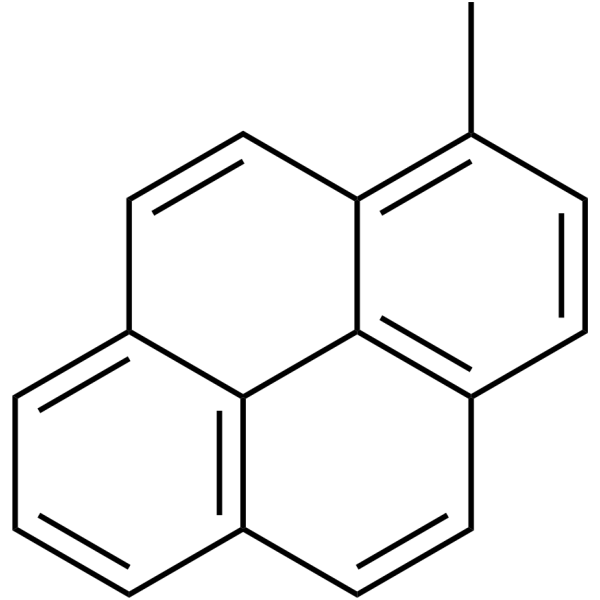
1-Methylpyrene
1-Methylpyrene structure
|
Common Name | 1-Methylpyrene | ||
|---|---|---|---|---|
| CAS Number | 2381-21-7 | Molecular Weight | 216.277 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 387.4±9.0 °C at 760 mmHg | |
| Molecular Formula | C17H12 | Melting Point | 72-74 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 178.9±12.8 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Nitroanilines as quenchers of pyrene fluorescence.
ChemPhysChem 13(18) , 4195-201, (2012) The quenching of pyrene and 1-methylpyrene fluorescence by nitroanilines (NAs), such as 2-, 3-, and 4-nitroaniline (2-NA, 3-NA, and 4-NA, respectively), 4-methyl-3-nitroaniline (4-M-3-NA), 2-methyl-4-nitroaniline (2-M-4-NA), and 4-methyl-3,5-dinitroaniline (4... |
|
|
Polycyclic aromatic hydrocarbons and the diffuse interstellar bands: a survey.
Astrophys. J. 526 Pt 1 , 265-73, (1999) We discuss the proposal relating the origin of some of the diffuse interstellar bands (DIBs) to neutral and ionized polycyclic aromatic hydrocarbons (PAHs) present in interstellar clouds. Laboratory spectra of several PAHs, isolated at low temperature in iner... |
|
|
1-Sulfooxymethylpyrene is an electrophilic mutagen and ultimate carcinogen of 1-methyl- and 1-hydroxymethylpyrene.
Biochem. Biophys. Res. Commun. 228(1) , 105-9, (1996) 1-Hydroxymethylpyrene and 1-sulfooxymethylpyrene were tested for complete carcinogenic activity by repeated s.c. injection in groups of 12 female Sprague-Dawley rats, respectively. A dose of 0.2 mumol of either 1-sulfooxymethylpyrene or 1-hydroxymethylpyrene ... |
|
|
Benzylic hydroxylation of 1-methylpyrene and 1-ethylpyrene by human and rat cytochromes P450 individually expressed in V79 Chinese hamster cells.
Carcinogenesis 20(9) , 1777-85, (1999) Alkyl-substituted polycyclic aromatic hydrocarbons may be metabolized to highly reactive benzylic sulfuric acid esters via benzylic hydroxylation and subsequent sulfonation. We have studied the benzylic hydroxylation of 1-methylpyrene (MP), a hepatocarcinogen... |
|
|
Methylated derivatives of pyrene and fluorene: evaluation of genotoxicity in the hepatocyte/DNA repair test and tumorigenic activity in newborn mice.
J. Toxicol. Environ. Health A 21(4) , 525-32, (1987) The genotoxicity of 1-methylpyrene, 1,6-dimethylpyrene, 1-methylfluorene, 9-methylfluorene, and 1,9-dimethylfluorene was evaluated in the hepatocyte primary culture/DNA repair test, and the tumorigenic potency of these compounds was tested by bioassay in newb... |
|
|
Time course of hepatic 1-methylpyrene DNA adducts in rats determined by isotope dilution LC-MS/MS and 32P-postlabeling.
Chem. Res. Toxicol. 21(10) , 2017-25, (2008) The alkylated polycyclic aromatic hydrocarbon 1-methylpyrene is a carcinogen in rodents and has been detected in various environmental matrices and foodstuffs. It is activated metabolically by benzylic hydroxylation to 1-hydroxymethylpyrene followed by sulfoc... |
|
|
Cytokine release and cytotoxicity in human keratinocytes induced by polycyclic aromatic hydrocarbons (1-methylpyrene and perylene).
J. Toxicol. Environ. Health A 73(8) , 552-64, (2010) The cytotoxic effects of two polycyclic aromatic hydrocarbons (PAH) (1-methyplyrene and perylene) were investigated on human skin keratinocytes. Normal human keratinocytes were cultured in the presence of various concentrations of 1-methylpyrene and perylene ... |
|
|
Mutation in Escherichia coli and mammalian cells induced by closely spaced 1-methylpyrene-deoxyadenosine adducts in opposite DNA strands.
Carcinogenesis 14(4) , 645-51, (1993) Twenty-eight base complementary oligonucleotides were synthesized with deoxyadenosine residues modified at the N6 position with 1-methylpyrene (MP) specifically positioned 3 bp apart in opposite DNA strands. Doubly modified constructs as well as non-modified ... |
|
|
Conformation of human fibrinogen in solution from polarized triplet spectroscopy.
Biochemistry 31(33) , 7580-6, (1992) The rotational motions of human fibrinogen in solution at 20 degrees C have been examined, in the 0.2-12-microseconds time range, by measuring the laser-induced dichroism of the triplet state of an erythrosin probe covalently bonded to the protein. The decay ... |
|
|
N. Boens et al.
Chem. Phys. Lett. 146 , 337, (1988)
|