4-Iodobenzoyl chloride
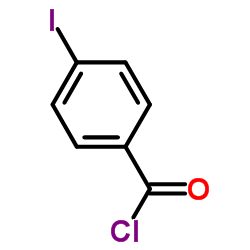
4-Iodobenzoyl chloride structure
|
Common Name | 4-Iodobenzoyl chloride | ||
|---|---|---|---|---|
| CAS Number | 1711-02-0 | Molecular Weight | 266.464 | |
| Density | 1.9±0.1 g/cm3 | Boiling Point | 310.6±0.0 °C at 760 mmHg | |
| Molecular Formula | C7H4ClIO | Melting Point | 62-66 °C | |
| MSDS | Chinese USA | Flash Point | 120.7±22.6 °C | |
| Symbol |

GHS05 |
Signal Word | Danger | |
|
Synthesis and Photophysical Studies of Calixarene-Based Alkynylplatinum(II) Terpyridine Complexes with Various Receptor Sites for Colorimetric and Luminescence Sensing of Anions.
Chemistry 22 , 3738-49, (2016) A series of mononuclear and dinuclear platinum(II) terpyridine complexes with amide-, sulfonamide-, and urea-containing ligands (1-9) has been successfully designed and synthesized, and their photophysical and anion-binding properties have been studied. The a... |
|
|
Direct synthesis of pyrroles from imines, alkynes, and acid chlorides: an isocyanide-mediated reaction.
Org. Lett. 9 , 449, (2007) [reaction: see text] A direct synthesis of pyrroles from imines, acid chlorides, and alkynes mediated by isocyanides is reported. This reaction proceeds with a range of each of these three substrates, providing a method to generate families of pyrroles in hig... |
|
|
Synthesis and characterization of novel radiopaque poly(allyl amine) nanoparticles.
Nanotechnology 21(33) , 335603, (2010) Contrast agents are currently used in a variety of diagnostic imaging techniques, including computer tomography for early cancer detection. Radiopaque nanoparticles have recently been proposed as an alternative method to traditional contrast agents that may a... |
|
|
N-(1-Benzylpyrrolidin-3-yl)arylbenzamides as potent and selective human dopamine D4 antagonists.
Bioorg. Med. Chem. Lett. 14(19) , 4847-50, (2004) A series of N-(1-benzylpyrrolidin-3-yl)arylbenzamides 8 has been prepared, and their structure-activity relationships studied. Potent ligands selective for human D(4) (hD(4)) over hD(2) and alpha(1) have been identified. One example was determined to be an an... |
|
|
Synthesis of poly [2] rotaxane by Sonogashira polycondensation. Sasabe H, et al.
J. Polym. Sci. A Polym. Chem. 45(17) , 4154-60, (2007)
|