3,3,3-Trifluoroalanine
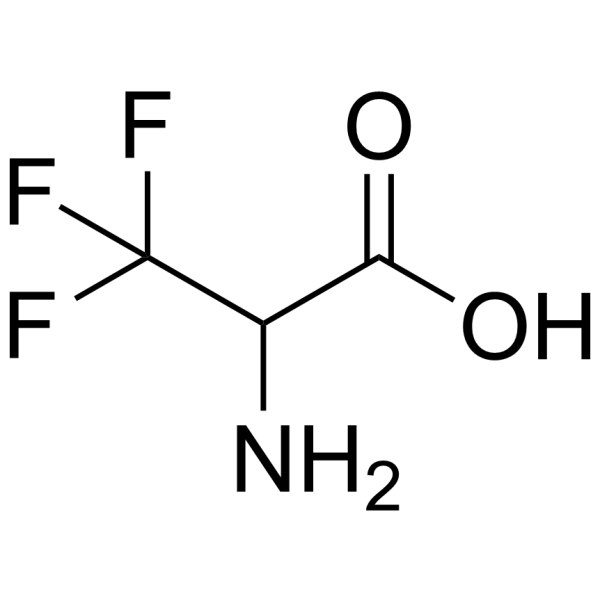
3,3,3-Trifluoroalanine structure
|
Common Name | 3,3,3-Trifluoroalanine | ||
|---|---|---|---|---|
| CAS Number | 17463-43-3 | Molecular Weight | 143.065 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 227.9±40.0 °C at 760 mmHg | |
| Molecular Formula | C3H4F3NO2 | Melting Point | 231-234ºC | |
| MSDS | Chinese USA | Flash Point | 91.6±27.3 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Low energy (0-10 eV) electron driven reactions in the halogenated organic acids CCl3COOH, CClF2COOH, and CF3CHNH2COOH (trifluoroalanine).
J. Chem. Phys. 135(12) , 124307, (2011) Negative ion formation following resonant electron attachment to the three title molecules is studied by means of a beam experiment with mass spectrometric detection of the anions. All three molecules exhibit a pronounced resonance in the energy range around ... |
|
|
Crystal structure of the pyridoxal-5'-phosphate dependent cystathionine beta-lyase from Escherichia coli at 1.83 A.
J. Mol. Biol. 262(2) , 202-24, (1996) Cystathionine beta-lyase (CBL) is a member of the gamma-family of PLP-dependent enzymes, that cleaves C beta-S bonds of a broad variety of substrates. The crystal structure of CBL from E. coli has been solved using MIR phases in combination with density modif... |
|
|
Characteristics of beta, beta-difluoroalanine and beta, beta, beta -trifluoroalanine as suicide substrates for Escherichia coli B alanine racemase.
Biochemistry 20(26) , 7539-46, (1981) The alanine racemase from Escherichia coli B has been shown to process DL isomers of beta -fluoroalanine as suicide substrates with an identical partitioning ratio for each enantiomer of 820 catalytic eliminations of HF per enzymatic inactivation event [Wang,... |
|
|
A novel route to dipeptides via noncondensation of amino acids: 2-aminoperfluoropropene as a synthon for trifluoroalanine dipeptides.
Org. Lett. 8(5) , 827-9, (2006) 2-Aminoperfluoropropene has been prepared by the Mg-promoted defluorinative N-silylation of N-p-methoxyphenyl hexafluoroacetone imine and has been employed as a synthon of trifluoroalanine for the preparation of trifluoroalanine dipeptides. |
|
|
Mechanism of inactivation of alanine racemase by beta, beta, beta-trifluoroalanine.
Biochemistry 28(2) , 431-7, (1989) The alanine racemases are a group of PLP-dependent bacterial enzymes that catalyze the racemization of alanine, providing D-alanine for cell wall synthesis. Inactivation of the alanine racemases from the Gram-negative organism Salmonella typhimurium and Gram-... |
|
|
Identification of the active-site residue of gamma-cystathionase labeled by the suicide inactivator beta, beta, beta-trifluoroalanine.
Biochemistry 21(16) , 3790-4, (1982) Inactivation of gamma-cystathionase by beta, beta, beta-trifluoroalanine, a suicide inactivator of the enzyme, results in covalent labeling of an amino group of the protein [Silverman, R. B., & Abeles, R. H. (1977) Biochemistry 16, 5515-5520]. We have establi... |
|
|
Identification and role of ionizing functional groups at the active center of Rhodotorula gracilis D-amino acid oxidase.
FEBS Lett. 507(3) , 323-6, (2001) D-Amino acid oxidase (DAAO) is a flavoprotein oxidase that catalyzes the oxidation of amino acids and produces ketoacids and H(2)O(2). The rate of product release from reduced DAAO from Rhodotorula gracilis is pH dependent and reflects a pK(a) of approximatel... |
|
|
Indole protects tryptophan indole-lyase, but not tryptophan synthase, from inactivation by trifluoroalanine.
Arch. Biochem. Biophys. 296(2) , 489-96, (1992) Trifluoroalanine is a mechanism-based inactivator of Escherichia coli tryptophan indole-lyase (tryptophanase) and E. coli tryptophan synthase (R. B. Silverman and R. H. Abeles, 1976, Biochemistry 15, 4718-4723). We have found that indole is able to prevent in... |