D-alanine
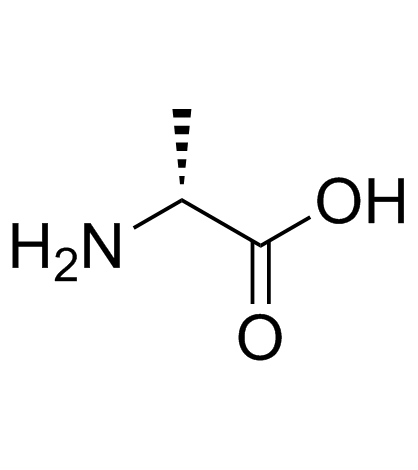
D-alanine structure
|
Common Name | D-alanine | ||
|---|---|---|---|---|
| CAS Number | 338-69-2 | Molecular Weight | 89.093 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 212.9±23.0 °C at 760 mmHg | |
| Molecular Formula | C3H7NO2 | Melting Point | 278-282ºC | |
| MSDS | Chinese USA | Flash Point | 82.6±22.6 °C | |
|
Evaluation of amino acid ester-based ionic liquids as buffer additives in CE for the separation of 2-arylpropionic acids nonsteroidal anti-inflammatory drugs.
Electrophoresis 35(18) , 2573-8, (2014) The aim of the present study is the CE performance evaluation for the separation of 2-arylpropionic acid nonsteroidal anti-inflammatory drugs. In particular, the separation of indoprofen, carprofen, ketoprofen, ibuprofen, and flurbiprofen was obtained by supp... |
|
|
A Novel Microbisporicin Producer Identified by Early Dereplication during Lantibiotic Screening.
Biomed Res. Int. 2015 , 419383, (2015) With the increasing need of effective antibiotics against multi-drug resistant pathogens, lantibiotics are an attractive option of a new class of molecules. They are ribosomally synthetized and posttranslationally modified peptides possessing potent antimicro... |
|
|
Knockout of the alanine racemase gene in Aeromonas hydrophila HBNUAh01 results in cell wall damage and enhanced membrane permeability.
FEMS Microbiol. Lett. 362 , fnv089, (2015) This study focused on the alanine racemase gene (alr-2), which is involved in the synthesis of d-alanine that forms the backbone of the cell wall. A stable alr-2 knockout mutant of Aeromonas hydrophila HBNUAh01 was constructed. When the mutant was supplemente... |
|
|
Salmonella evades D-amino acid oxidase to promote infection in neutrophils.
MBio 5(6) , e01886, (2014) Neutrophils engulf and kill bacteria using oxidative and nonoxidative mechanisms. Despite robust antimicrobial activity, neutrophils are impaired in directing Salmonella clearance and harbor viable intracellular bacteria during early stages of infection that ... |
|
|
Factor-inhibiting HIF-1 (FIH-1) is required for human vascular endothelial cell survival.
FASEB J. 29 , 2814-27, (2015) Factor-inhibiting hypoxia-inducible factor (HIF)-1 (FIH-1) is an asparaginyl β-hydroxylase enzyme that was initially found to hydroxylate the HIF-α, preventing its transcriptional activity and leading to adaptive responses to hypoxia. More recently, other sub... |
|
|
A putative low-molecular-mass penicillin-binding protein (PBP) of Mycobacterium smegmatis exhibits prominent physiological characteristics of DD-carboxypeptidase and beta-lactamase.
Microbiology 161 , 1081-91, (2015) DD-carboxypeptidases (DD-CPases) are low-molecular-mass (LMM) penicillin-binding proteins (PBPs) that are mainly involved in peptidoglycan remodelling, but little is known about the dd-CPases of mycobacteria. In this study, a putative DD-CPase of Mycobacteriu... |
|
|
Monitoring neonatal fungal infection with metabolomics.
J. Matern. Fetal. Neonatal. Med. 27 Suppl 2 , 34-8, (2014) The objective of our study was to evaluate the capability of the metabolomics approach to identify the variations of urine metabolites over time related to the neonatal fungal septic condition. The study population included a clinical case of a preterm neonat... |
|
|
Regulation of Clostridium difficile Spore Formation by the SpoIIQ and SpoIIIA Proteins.
PLoS Genet. 11 , e1005562, (2015) Sporulation is an ancient developmental process that involves the formation of a highly resistant endospore within a larger mother cell. In the model organism Bacillus subtilis, sporulation-specific sigma factors activate compartment-specific transcriptional ... |
|
|
Three-dimensional quantitative structure-activity relationship analyses of substrates of the human proton-coupled amino acid transporter 1 (hPAT1).
Bioorg. Med. Chem. 19 , 6409-18, (2011) The proton-coupled amino acid transporter hPAT1 has recently gained much interest due to its ability to transport small drugs thereby allowing their oral administration. A three-dimensional quantitative structure-activity relationship (3D QSAR) study has been... |
|
|
Drug design, in vitro pharmacology, and structure-activity relationships of 3-acylamino-2-aminopropionic acid derivatives, a novel class of partial agonists at the glycine site on the N-methyl-D-aspartate (NMDA) receptor complex.
J. Med. Chem. 52 , 5093-107, (2009) Retaining agonistic activity at the glycine coagonist site of the NMDA receptor in molecules derived from glycine or d-serine has proven to be difficult because in the vicinity of the alpha-amino acid group little substitution is tolerated. We have solved thi... |