Sulindac Sulfide
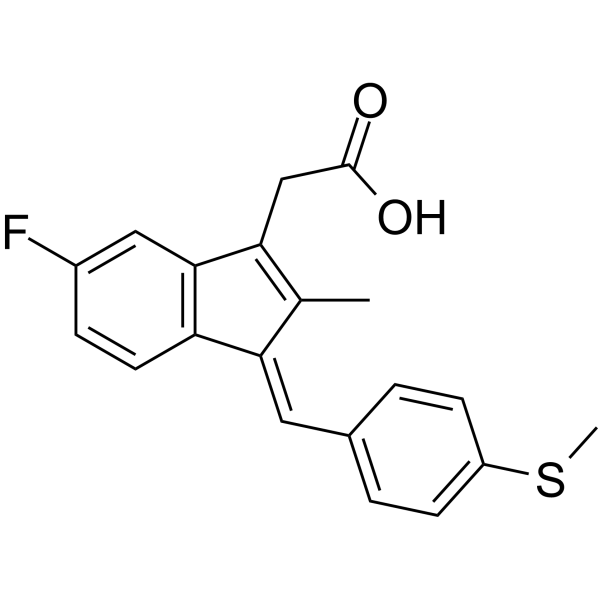
Sulindac Sulfide structure
|
Common Name | Sulindac Sulfide | ||
|---|---|---|---|---|
| CAS Number | 32004-67-4 | Molecular Weight | 340.411 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 526.3±50.0 °C at 760 mmHg | |
| Molecular Formula | C20H17FO2S | Melting Point | 189-191°C | |
| MSDS | Chinese USA | Flash Point | 272.1±30.1 °C | |
| Symbol |


GHS07, GHS08 |
Signal Word | Danger | |
|
Capping of aβ42 oligomers by small molecule inhibitors.
Biochemistry 53(50) , 7893-903, (2014) Aβ42 peptides associate into soluble oligomers and protofibrils in the process of forming the amyloid fibrils associated with Alzheimer's disease. The oligomers have been reported to be more toxic to neurons than fibrils, and have been targeted by a wide rang... |
|
|
Potential for drug interactions mediated by polymorphic flavin-containing monooxygenase 3 in human livers.
Drug Metab. Pharmacokinet. 30(1) , 70-4, (2015) Human flavin-containing monooxygenase 3 (FMO3) in the liver catalyzes a variety of oxygenations of nitrogen- and sulfur-containing medicines and xenobiotic substances. Because of growing interest in drug interactions mediated by polymorphic FMO3, benzydamine ... |
|
|
Sulindac sulfide--induced stimulation of eryptosis.
Cell Physiol. Biochem. 30(4) , 1072-82, (2012) Sulindac sulfide, a non-steroidal anti-inflammatory drug (NSAID), stimulates apoptosis of tumor cells and is thus effective against malignancy. In analogy to apoptosis of nucleated cells, erythrocytes may undergo eryptosis, an apoptosis-like suicidal erythroc... |
|
|
Neuritogenic Activity of Tetradecyl 2,3-Dihydroxybenzoate Is Mediated through the Insulin-Like Growth Factor 1 Receptor/Phosphatidylinositol 3 Kinase/Mitogen-Activated Protein Kinase Signaling Pathway.
Mol. Pharmacol. 88 , 326-34, (2015) Tetradecyl 2,3-dihydroxybenzoate (ABG-001) is a lead compound derived from neuritogenic gentisides. In the present study, we investigated the mechanism by which ABG-001 induces neurite outgrowth in a rat adrenal pheochromocytoma cell line (PC12). Inhibitors o... |
|
|
Entrapment of human flavin-containing monooxygenase 3 in the presence of gold nanoparticles: TEM, FTIR and electrocatalysis.
Biochim. Biophys. Acta 1820(12) , 2072-8, (2012) Nanosized particles of gold are widely used as advanced materials for enzyme catalysis investigations. In some bioanalytical methods these nanoparticles can be exploited to increase the sensitivity by enhancing electron transfer to the biological component i.... |
|
|
CTF1-51, a truncated carboxyl-terminal fragment of amyloid precursor protein, suppresses the effects of Aβ42-lowering γ-secretase modulators.
Neurosci. Lett. 526(2) , 96-9, (2012) The pathogenesis of Alzheimer's disease (AD) is correlated with the toxicity of amyloid β-peptide (Aβ), especially Aβ42. γ-Secretase modulators (GSMs) are compounds that alter production of Aβ42 without interfering with the physiological function of γ-secreta... |
|
|
Sulindac activates NF-κB signaling in colon cancer cells.
Cell Commun. Signal. 11 , 73, (2013) The non-steroidal anti-inflammatory drug (NSAID) sulindac has shown efficacy in preventing colorectal cancer. This potent anti-tumorigenic effect is mediated through multiple cellular pathways but is also accompanied by gastrointestinal side effects, such as ... |
|
|
Anti-tumor activity of non-steroidal anti-inflammatory drugs: cyclooxygenase-independent targets.
Cancer Lett. 346(2) , 217-24, (2014) Non-steroidal anti-inflammatory drugs (NSAIDs) are used extensively for analgesic and antipyretic treatments. In addition, NSAIDs reduce the risk and mortality to several cancers. Their mechanisms in anti-tumorigenesis are not fully understood, but both cyclo... |
|
|
Second generation γ-secretase modulators exhibit different modulation of Notch β and Aβ production.
J. Biol. Chem. 287(39) , 32640-50, (2012) The γ-secretase complex is an appealing drug target when the therapeutic strategy is to alter amyloid-β peptide (Aβ) aggregation in Alzheimer disease. γ-Secretase is directly involved in Aβ formation and determines the pathogenic potential of Aβ by generating... |
|
|
Drug oxygenation activities mediated by liver microsomal flavin-containing monooxygenases 1 and 3 in humans, monkeys, rats, and minipigs.
Biochem. Pharmacol. 90(2) , 159-65, (2014) Liver microsomal flavin-containing monooxygenases (FMO, EC 1.14.13.8) 1 and 3 were functionally characterized in terms of expression levels and molecular catalytic capacities in human, cynomolgus monkey, rat, and minipig livers. Liver microsomal FMO3 in human... |